Appendix VIII L. Residual Solvents
The test procedures described in this general method may be used:
i. for the identification of the majority of Class 1 and Class 2 residual solvents in an active substance, excipient or medicinal product when the residual solvents are unknown;
ii. as a limit test for Class 1 and Class 2 solvents when present in an active substance, excipient or medicinal product;
iii. for the quantification of Class 2 solvents when the limits are greater than 1000 ppm (0.1 per cent) or for the quantification of Class 3 solvents when required.
Class 1, Class 2 and Class 3 residual solvents are listed in general chapter 5.4. Residual solvents.
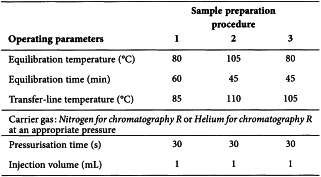
Three diluents are described for sample preparation and the conditions to be applied for head-space injection of the gaseous sample onto the chromatographic system. Two chromatographic systems are prescribed but System A is preferred whilst System B is employed normally for confirmation of identity. The choice of sample preparation procedure depends on the solubility of the substance to be examined and in certain cases the residual solvents to be controlled.
The following residual solvents are not readily detected by the head-space injection conditions described: formamide, 2-ethoxyethanol, 2-methoxyethanol, ethylene glycol, N-methylpyrrolidone and sulfolane. Other appropriate procedures should be employed for the control of these residual solvents.
When the test procedure is applied quantitatively to control residual solvents in a substance, then it must be validated.
PROCEDURE
Examine by gas chromatography with static head-space injection (2.2.28).
Sample preparation 1
This is intended for the control of residual solvents in water-soluble substances.
Sample solution (1) Dissolve 0.200 g of the substance to be examined in water R and dilute to 20.0 mL with the same solvent.
Sample preparation 2
This is intended for the control of residual solvents in water-insoluble substances.
Sample solution (2) Dissolve 0.200 g of the substance to be examined in dimethylformamide R (DMF) and dilute to 20.0 mL with the same solvent.
Sample preparation 3
This is intended for the control of N,N-dimethylacetamide and/or N,N-dimethylformamide, when it is known or suspected that one or both of these substances are present in the substance to be examined.
Sample solution (3) Dissolve 0.200 g of the substance to be examined in 1,3-dimethyl-2-imidazolidinone R (DMI) and dilute to 20.0 mL with the same solvent.
In some cases none of the above sample preparation procedures are appropriate, in which case the diluent to be used for the preparation of the sample solution and the static head-space conditions to be employed must be demonstrated to be suitable.
Solvent solution (a) To 1.0 mL of Class 1 residual solvent solution CRS, add 9 mL of dimethyl sulfoxide R and dilute to 100.0 mL with water R. Dilute 1.0 mL of this solution to 100 mL with water R. Dilute 1.0 mL of this solution to 10.0 mL with water R.
The reference solutions correspond to the following limits:
Solvent solution (b) Dissolve appropriate quantities of the Class 2 residual solvents in dimethyl sulfoxide R and dilute to 100.0 mL with the same solvent. Dilute in water R to give a concentration of 1/20 of the limits stated in Table 2 (see 5.4. Residual solvents).
Solvent solution (c) Dissolve 1.00 g of the solvent or solvents present in the substance to be examined in dimethyl sulfoxide R or water R, if appropriate, and dilute to 100.0 mL with water R. Dilute to give a concentration of 1/20 of the limit(s) stated in Table 1 or 2 (see 5.4. Residual solvents).
Blank solution Prepare as described for solvent solution (c) but without the addition of solvent(s) (used to verify the absence of interfering peaks).
Test solution Introduce 5.0 mL of the sample solution and 1.0 mL of the blank solution into an injection vial.
Reference solution (a) (Class 1) Introduce 1.0 mL of solvent solution (a) and 5.0 mL of the appropriate diluent into an injection vial.
Reference solution (a) (Class 1)1 Introduce 5.0 mL of the sample solution and 1.0 mL of solvent solution (a) into an injection vial.
Reference solution (b) (Class 2) Introduce 1.0 mL of solvent solution (b) and 5.0 mL of the appropriate diluent into an injection vial.
Reference solution (c) Introduce 5.0 mL of the sample solution and 1.0 mL of solvent solution (c) into an injection vial.
Reference solution (d) Introduce 1.0 mL of the blank solution and 5.0 mL of the appropriate diluent into an injection vial.
Close the vials with a tight rubber membrane stopper coated with polytetrafluoroethylene and secure with an aluminium crimped cap. Shake to obtain a homogeneous solution.
The following static head-space injection conditions may be used:
The chromatographic procedure may be carried out using:
System A
maintaining the temperature of the column at 40 °C for 20 min, then raising the temperature at a rate of 10 °C per min to 240 °C and maintaining it at 240 °C for 20 min and maintaining the temperature of the injection port at 140 °C and that of the detector at 250 °C, or, where there is interference from the matrix, use:
System B
maintaining the temperature of the column at 50 °C for 20 min, then raising the temperature at a rate of 6 °C per min to 165 °C and maintaining it at 165 °C for 20 min and maintaining the temperature of the injection port at 140 °C and that of the detector at 250 °C.
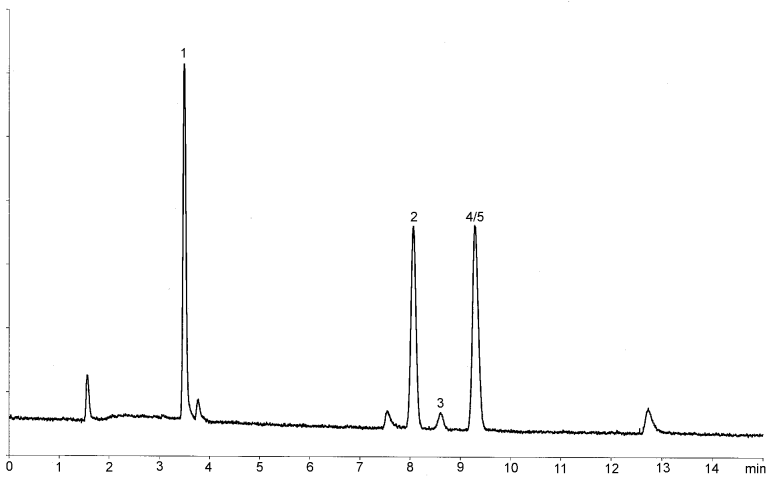
Inject 1 mL of the gaseous phase of reference solution (a) onto the column described in System A and record the chromatogram under such conditions that the signal-to-noise ratio for 1,1,1-trichloroethane can be measured. The signal-to-noise ratio must be at least 5. A typical chromatogram is shown in Figure 2.4.24.-1.
Inject 1 mL of the gaseous phase of reference solution (a1) onto the column described in System A. The peaks due to the Class 1 residual solvents are still detectable.
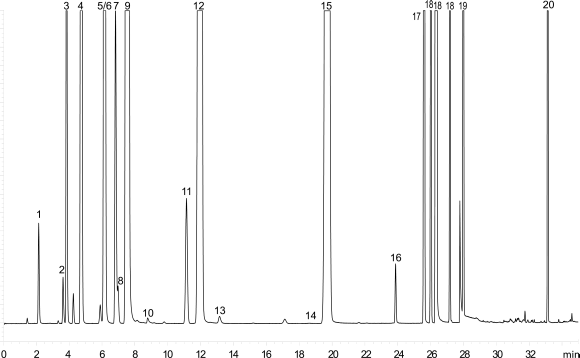
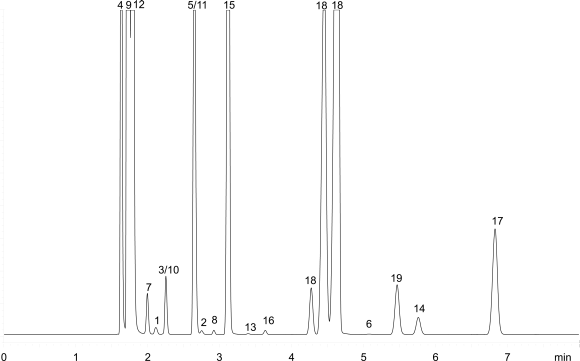
Inject 1 mL of the gaseous phase of reference solution (b) onto the column described in System A and record the chromatogram under such conditions that the resolution between acetonitrile and methylene chloride can be determined. The system is suitable if the chromatogram obtained resembles the chromatogram shown in Figure 2.4.24.-2 and the resolution between acetonitrile and methylene chloride is at least 1.0.
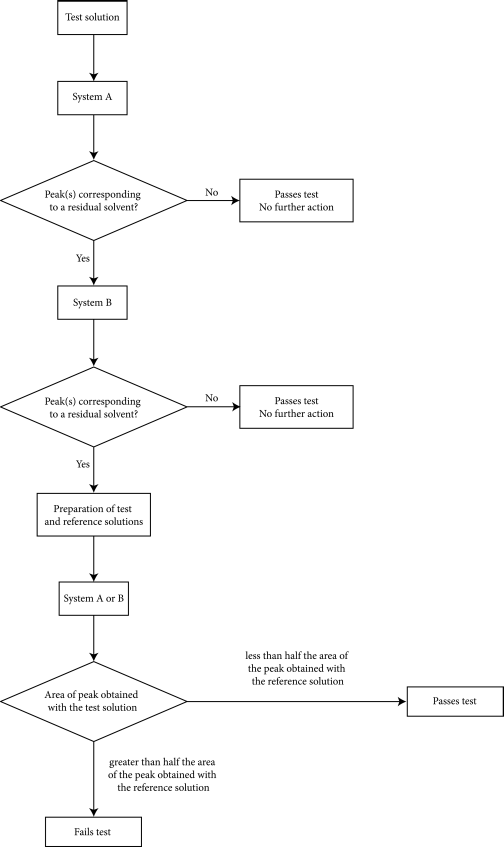
Inject 1 mL of the gaseous phase of the test solution onto the column described in System A. If in the chromatogram obtained, there is no peak which corresponds to one of the residual solvent peaks in the chromatograms obtained with reference solution (a) or (b), then the substance to be examined meets the requirements of the test. If any peak in the chromatogram obtained with the test solution corresponds to any of the residual solvent peaks obtained with reference solution (a) or (b) then System B is to be employed.
Inject 1 mL of the gaseous phase of reference solution (a) onto the column described in System B and record the chromatogram under such conditions that the signal-to-noise ratio for benzene can be measured. The signal-to-noise ratio must be at least 5. A typical chromatogram is shown in Figure 2.4.24.-3.
Inject 1 mL of the gaseous phase of reference solution (a1) onto the column described in System B. The peaks due to the Class 1 residual solvents are still detectable.
Inject 1 mL of the gaseous phase of reference solution (b) onto the column described in System B and record the chromatogram under such conditions that the resolution between acetonitrile and trichloroethene can be determined. The system is suitable if the chromatogram obtained resembles the chromatogram shown in Figure 2.4.24.-4 and the resolution between acetonitrile and trichloroethene is at least 1.0.
Inject 1 mL of the gaseous phase of the test solution onto the column described in System B. If in the chromatogram obtained, there is no peak which corresponds to any of the residual solvent peaks in the chromatogram obtained with the reference solution (a) or (b), then the substance to be examined meets the requirements of the test. If any peak in the chromatogram obtained with the test solution corresponds to any of the residual solvent peaks obtained with reference solution (a) or (b) and confirms the correspondence obtained when using System A, then proceed as follows.
Inject 1 mL of the gaseous phase of reference solution (c) onto the column described for System A or System B. If necessary, adjust the sensitivity of the system so that the height of the peak corresponding to the identified residual solvent(s) is at least 50 per cent of the full scale of the recorder.
Inject 1 mL of the gaseous phase of reference solution (d) onto the column. No interfering peaks should be observed.
Inject 1 mL of the gaseous phase of the test solution and 1 mL of the gaseous phase of reference solution (c) on to the column. Repeat these injections twice more.
The mean area of the peak of the residual solvent(s) in the chromatograms obtained with the test solution is not greater than half the mean area of the peak of the corresponding residual solvent(s) in the chromatograms obtained with reference solution (c). The test is not valid unless the relative standard deviation of the differences in areas between the analyte peaks obtained from 3 replicate paired injections of reference solution (c) and the test solution, is at most 15 per cent.
A flow diagram of the procedure is shown in Figure 2.4.24.-5.
When a residual solvent (Class 2 or Class 3) is present at a level of 0.1 per cent or greater then the content may be quantitatively determined by the method of standard additions.
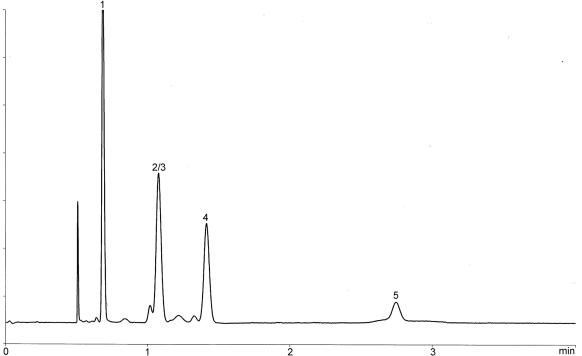
| 1. 1,1-dichloroethene | 2. 1,1,1-trichloroethane | 3. carbon tetrachloride | 4. benzene | 5. 1,2-dichloroethane |
| 1. methanol | 5. 1,2-dichloroethene | 9. cyclohexane | 13. 1,4-dioxane | 17. chlorobenzene | |
| 2.acetonitrile | 6. nitromethane | 10. 1,2-dimethoxyethane | 14. pyridine (tr = 18.7 min) | 18. xylene ortho, meta, para | |
| 3. dichloromethane | 11. 1,1,2-trichloroethene | 15. toluene | 19. cumene | ||
| 4. hexane | 8. chloroform | 12. methylcyclohexane | 16. methylbutylketone | 20. tetralin |
| 1. 1,1-dichloroethene | 2. 1,1,1-trichloroethane | 3. carbon tetrachloride | 4. benzene | 5. 1,2-dichloroethane |
| 1. methanol | 5. 1,2-dichloroethene | 9. cyclohexane | 13. 1,4-dioxane | 17. chlorobenzene | |
| 2.acetonitrile | 6. nitromethane | 10. 1,2-dimethoxyethane | 14. pyridine | 18. xylene ortho, meta, para | |
| 3. dichloromethane | 11. 1,1,2-trichloroethene | 15. toluene | 19. cumene | ||
| 4. hexane | 8. chloroform | 12. methylcyclohexane | 16. methylbutylketone | 20. tetralin (tr = 27 min) |