Nystatin
A to Z Drug Facts
| (nye-STAT-in) |
Mycostatin, Mycostatin Pastilles, Nilstat, Nystex, Pedi-Dri,  Candistatin, Nadostine, Nyaderm, PMS-Nystatin Candistatin, Nadostine, Nyaderm, PMS-Nystatin |
| Class: Anti-infective/Antifungal |
 Action Binds to fungal cell membrane, changing membrane permeability and allowing leakage of intracellular components.
Action Binds to fungal cell membrane, changing membrane permeability and allowing leakage of intracellular components.
 Indications Treatment of intestinal, oral, vulvovaginal, cutaneous, or mucocutaneous candidiasis.
Indications Treatment of intestinal, oral, vulvovaginal, cutaneous, or mucocutaneous candidiasis.
 Contraindications Standard considerations.
Contraindications Standard considerations.
 Route/Dosage
Route/Dosage
Intestinal Candidiasis
ADULTS and CHILDREN: PO 500,000 to 1,000,000 U tid. Continue treatment for ³ 48 hr after clinical cure.
Oral or Mucocutaneous Candidiasis
ADULTS & CHILDREN: PO (suspension) 200,000 to 600,000 U qid; swish and swallow, or PO (oral pastilles) 1 to 2 pastilles (200,000 to 400,000 U) dissolved in mouth 4 to 5 times/day. INFANTS: PO 200,000 U qid.
Vaginal Candidiasis
ADULTS: Intravaginal 100,000 U qd for 2 wk.
Cutaneous Candidiasis
ADULTS & CHILDREN: Topical Apply to affected areas bid to tid.
 Interactions None well documented.
Interactions None well documented.
 Lab Test Interferences None well documented.
Lab Test Interferences None well documented.
 Adverse Reactions
Adverse Reactions
GI: Diarrhea; GI distress; nausea; vomiting (with large oral doses). DERM: Irritation (with topical use).
 Precautions
Precautions
Pregnancy: Category C (oral); Category A (vaginal). Lactation: Undetermined. Effectiveness: Has no activity against bacteria or trichomonads. Not indicated for systemic mycoses. Topical preparations: Not for ophthalmic use.
| PATIENT CARE CONSIDERATIONS |
|
 Administration/Storage
Administration/Storage
- For troche/pastilles administration, have patient dissolve troche in mouth. Instruct patient not to chew or swallow troche whole. Have patient retain troche in mouth as long as possible before swallowing.
- For administration of oral suspension, place half of dose in each side of mouth. Have patient swish thoroughly around in mouth and retain in mouth as long as possible before swallowing. Shake suspension well. Use calibrated dropper provided.
- Do not mix oral suspension in foods.
- To use powder for extemporaneous compounding, reconstitute
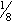 tsp (500,000 U) in half glass of water and stir well. Use immediately; do not store. Can be administered in form of flavored frozen popsicle.
tsp (500,000 U) in half glass of water and stir well. Use immediately; do not store. Can be administered in form of flavored frozen popsicle.
- Cream or ointment is preferred for affected intertriginous areas. Wash and dry affected area before application. Use gloves or swabs to apply enough medication to cover lesion completely.
- To use powder, clean and dry affected area before application. Dust powder on feet and in socks and shoes for infection of feet. Very moist lesions are best treated with powder.
- For vaginal use, insert 1 tablet high into vagina with applicator.
- Refrigerate vaginal tablets.
- Avoid contact with eyes.
- Store oral suspension in refrigerator. Protect from heat, light, moisture, and air.
 Assessment/Interventions
Assessment/Interventions
- Obtain patient history, including drug history and any known allergies.
- Although allergic reactions are rare, assess for rash, urticaria, burning, stinging, redness, and swelling.
- Assess for factors predisposing to infection: pregnancy, antibiotic therapy, diabetes, infected sexual partners (vaginal infections). However, vaginal yeast infections are common and may not be associated with any of these factors.
- Inspect mucous membranes before and frequently throughout course of therapy.
| OVERDOSAGE: SIGNS & SYMPTOMS |
| |
Nausea, diarrhea, vomiting |
|
 Patient/Family Education
Patient/Family Education
- Instruct patient that long-term therapy may be needed to clear infection and that patient should complete entire course of medication. Take drug for 2 days after symptoms have disappeared or as directed.
- Advise patient using vaginal preparations to wear light-day pad; drug may stain clothing and linens.
- Advise patient to notify physician if irritation occurs.
- Assure patient that relief from itching may occur after 24 to 72 hr.
- Instruct patient to practice good hand washing before and after each application of topical or vaginal medication. Remind patient to wash applicator after each use.
- Advise patient with oral candidiasis not to use mouthwash, which may alter normal flora and promote infections.
- Teach patient to continue using vaginal tablets even when menstruating because treatment should be continued for 2 wk. Instruct patient to avoid using tampons.
- Explain to patient that during pregnancy, use of vaginal applicator may be contraindicated, and manual insertion of vaginal tablets may be preferred.
- Advise patient to prevent reinfection (ie, avoid intercourse during therapy or use condoms).
- Instruct patient to discontinue drug and notify physician if vaginal tablets cause irritation, redness, or swelling.
Books@Ovid
Copyright © 2003 Facts and Comparisons
David S. Tatro
A to Z Drug Facts
 Candistatin, Nadostine, Nyaderm, PMS-Nystatin
Candistatin, Nadostine, Nyaderm, PMS-Nystatin Candistatin, Nadostine, Nyaderm, PMS-Nystatin
Candistatin, Nadostine, Nyaderm, PMS-Nystatin Action Binds to fungal cell membrane, changing membrane permeability and allowing leakage of intracellular components.
Action Binds to fungal cell membrane, changing membrane permeability and allowing leakage of intracellular components. Indications Treatment of intestinal, oral, vulvovaginal, cutaneous, or mucocutaneous candidiasis.
Indications Treatment of intestinal, oral, vulvovaginal, cutaneous, or mucocutaneous candidiasis. Contraindications Standard considerations.
Contraindications Standard considerations. Interactions None well documented.
Interactions None well documented. Lab Test Interferences None well documented.
Lab Test Interferences None well documented. tsp (500,000 U) in half glass of water and stir well. Use immediately; do not store. Can be administered in form of flavored frozen popsicle.
tsp (500,000 U) in half glass of water and stir well. Use immediately; do not store. Can be administered in form of flavored frozen popsicle.