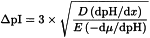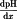Appendix III J. Isoelectric Focusing1
GENERAL PRINCIPLES
Isoelectric focusing (IEF) is a method of electrophoresis that separates proteins according to their isoelectric point. Separation is carried out in a slab of polyacrylamide or agarose gel that contains a mixture of amphoteric electrolytes (ampholytes). When subjected to an electric field, the ampholytes migrate in the gel to create a pH gradient. In some cases gels containing an immobilised pH gradient, prepared by incorporating weak acids and bases to specific regions of the gel network during the preparation of the gel, are used. When the applied proteins reach the gel fraction that has a pH that is the same as their isoelectric point (pI), their charge is neutralised and migration ceases. Gradients can be made over various ranges of pH, according to the mixture of ampholytes chosen.
THEORETICAL ASPECTS
When a protein is at the position of its isoelectric point, it has no net charge and cannot be moved in a gel matrix by the electric field. It may, however, move from that position by diffusion. The pH gradient forces a protein to remain in its isoelectric point position, thus concentrating it; this concentrating effect is called “focusing”. Increasing the applied voltage or reducing the sample load result in improved separation of bands. The applied voltage is limited by the heat generated, which must be dissipated. The use of thin gels and an efficient cooling plate controlled by a thermostatic circulator prevents the burning of the gel whilst allowing sharp focusing. The separation is estimated by determining the minimum pI difference (ΔpI), which is necessary to separate 2 neighbouring bands:
D | = | diffusion coefficient of the protein, |
= | pH gradient, | |
E | = | intensity of the electric field, in volts per centimetre, |
= | variation of the solute mobility with the pH in the region close to the pI. |
Since D and 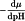 for a given protein cannot be altered, the separation can be improved by using a narrower pH range and by increasing the intensity of the electric field.
for a given protein cannot be altered, the separation can be improved by using a narrower pH range and by increasing the intensity of the electric field.
Resolution between protein bands on an IEF gel prepared with carrier ampholytes can be quite good. Improvements in resolution may be achieved by using immobilised pH gradients where the buffering species, which are analogous to carrier ampholytes, are copolymerised within the gel matrix. Proteins exhibiting pIs differing by as little as 0.02 pH units may be resolved using a gel prepared with carrier ampholytes while immobilised pH gradients can resolve proteins differing by approximately 0.001 pH units.
PRACTICAL ASPECTS
Special attention must be paid to sample characteristics and/or preparation. Having salt in the sample can be problematic and it is best to prepare the sample, if possible, in deionised water or 2 per cent ampholytes, using dialysis or gel filtration if necessary.
The time required for completion of focusing in thin-layer polyacrylamide gels is determined by placing a coloured protein (e.g. haemoglobin) at different positions on the gel surface and by applying the electric field: the steady state is reached when all applications give an identical band pattern. In some protocols the completion of the focusing is indicated by the time elapsed after the sample application.
The IEF gel can be used as an identity test when the migration pattern on the gel is compared to a suitable standard preparation and IEF calibration proteins, the IEF gel can be used as a limit test when the density of a band on IEF is compared subjectively with the density of bands appearing in a standard preparation, or it can be used as a quantitative test when the density is measured using a densitometer or similar instrumentation to determine the relative concentration of protein in the bands subject to validation.
APPARATUS
An apparatus for IEF consists of:
ISOELECTRIC FOCUSING IN POLYACRYLAMIDE GELS: DETAILED PROCEDURE
The following method is a detailed description of an IEF procedure in thick polyacrylamide slab gels, which is used unless otherwise stated in the monograph.
Preparation of the gels
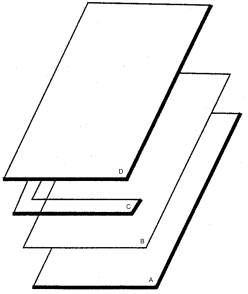
Mould
The mould (see Figure 2.2.54.-1) is composed of a glass plate (A) on which a polyester film (B) is placed to facilitate handling of the gel, one or more spacers (C), a second glass plate (D) and clamps to hold the structure together.
7.5 per cent polyacrylamide gel
Dissolve 29.1 g of acrylamide R and 0.9 g of methylenebisacrylamide R in 100 mL of water R. To 2.5 volumes of this solution, add the mixture of ampholytes specified in the monograph and dilute to 10 volumes with water R. Mix carefully and degas the solution.
Preparation of the mould
Place the polyester film on the lower glass plate, apply the spacer, place the second glass plate and fit the clamps. Before use, place the solution on a magnetic stirrer and add 0.25 volumes of a 100 g/L solution of ammonium persulfate R and 0.25 volumes of tetramethylethylenediamine R. Immediately fill the space between the glass plates of the mould with the solution.
Method
Dismantle the mould and, making use of the polyester film, transfer the gel onto the cooled support, wetted with a few millilitres of a suitable liquid, taking care to avoid forming air bubbles. Prepare the test solutions and reference solutions as specified in the monograph. Place strips of paper for sample application, about 10 mm × 5 mm in size, on the gel and impregnate each with the prescribed amount of the test and reference solutions. Also apply the prescribed quantity of a solution of proteins with known isoelectric points as pH markers to calibrate the gel. In some protocols the gel has pre-cast slots where a solution of the sample is applied instead of using impregnated paper strips. Cut 2 strips of paper to the length of the gel and impregnate them with the electrolyte solutions: acid for the anode and alkaline for the cathode. The compositions of the anode and cathode solutions are given in the monograph. Apply these paper wicks to each side of the gel several millimetres from the edge. Fit the cover so that the electrodes are in contact with the wicks (respecting the anodic and cathodic poles). Proceed with the isoelectric focusing by applying the electrical parameters described in the monograph. Switch off the current when the migration of the mixture of standard proteins has stabilised. Using forceps, remove the sample application strips and the 2 electrode wicks. Immerse the gel in fixing solution for isoelectric focusing in polyacrylamide gel R. Incubate with gentle shaking at room temperature for 30 min. Drain off the solution and add 200 mL of destaining solution R. Incubate with shaking for 1 h. Drain the gel, add coomassie staining solution R. Incubate for 30 min. Destain the gel by passive diffusion with destaining solution R until the bands are well visualised against a clear background. Locate the position and intensity of the bands in the electropherogram as prescribed in the monograph.
VARIATIONS TO THE DETAILED PROCEDURE (SUBJECT TO VALIDATION)
Where reference to the general method on isoelectric focusing is made, variations in methodology or procedure may be made subject to validation. These include:
VALIDATION OF ISO-ELECTRIC FOCUSING PROCEDURES
Where alternative methods to the detailed procedure are employed they must be validated. The following criteria may be used to validate the separation:
SPECIFIED VARIATIONS TO THE GENERAL METHOD
Variations to the general method required for the analysis of specific substances may be specified in detail in monographs. These include:
POINTS TO CONSIDER
Samples can be applied to any area on the gel, but to protect the proteins from extreme pH environments samples should not be applied close to either electrode. During method development the analyst can try applying the protein in 3 positions on the gel (i.e. middle and both ends); the pattern of a protein applied at opposite ends of the gel may not be identical.
A phenomenon known as cathodic drift, where the pH gradient decays over time, may occur if a gel is focused too long. Although not well understood, electroendoosmosis and absorption of carbon dioxide may be factors that lead to cathodic drift. Cathodic drift is observed as focused protein migrating off the cathode end of the gel. Immobilised pH gradients may be used to address this problem.
Efficient cooling (approximately 4 °C) of the bed that the gel lies on during focusing is important. High field strengths used during isoelectric focusing can lead to overheating and affect the quality of the focused gel.