SC IV F. Pharmacopoeial Harmonisation
This general chapter is included for guidance of users.
It provides information on the degree of harmonisation of various general chapters and monographs of the European Pharmacopoeia and those of the Japanese Pharmacopoeia and United States Pharmacopeia.
This information reflects:
However, it remains the ultimate responsibility of the user to verify the current content of the texts in force in the respective pharmacopoeias.
The chapter does not affect in any way the status of the monographs and general chapters as the authoritative reference in any case of doubt or dispute where compliance with the European Pharmacopoeia is required.
The European Pharmacopoeia Commission recognises the utility of working with other pharmacopoeial bodies to develop harmonised monographs and general chapters. Such harmonisation is fully compatible with the declared aims of the Commission and has benefits of different kinds, notably the simplification and rationalisation of quality control methods and licensing procedures. Such harmonisation also enhances the benefits of the work of ICH and the International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH), since some of the guidelines developed depend on pharmacopoeial general chapters for their application.
Work on harmonisation is carried out by a well-defined but informal process in the Pharmacopoeial Discussion Group (PDG), in which the European Pharmacopoeia, the Japanese Pharmacopoeia and the United States Pharmacopeia are associated. Information is given in this general chapter on items that have been dealt with by the PDG:
Information on any non-harmonised attributes/provisions and on any local requirements, i.e. attributes/provisions that are present only in the Ph. Eur. text, is included in this general chapter. The non-harmonised attributes/provisions are placed between black diamonds (♦♦) in the corresponding Ph. Eur. texts, while the local requirements are placed between white diamonds (♢♢).
The non-mandatory Functionality-related characteristics section is specific to the Ph. Eur.; it is not subject to pharmacopoeial harmonisation and is therefore not placed between black or white diamonds.
The 3 pharmacopoeias have undertaken not to make unilateral changes to harmonised monographs and general chapters but rather to apply the agreed revision procedure whereby all partners adopt a revision simultaneously.
General Chapters
The documents signed-off by the PDG cover the technical content of the text and each party adapts them as necessary to conform to the usual presentation of the pharmacopoeia in question; such adaptation includes stipulation of the particular pharmacopoeia′s reference materials and general chapters.
2.2.31. Electrophoresis
The following comparative commentary refers to the texts 23. SDS-Polyacrylamide Gel Electrophoresis in the Japanese Pharmacopoeia XV and <1056> Biotechnology-derived Articles – Polyacrylamide Gel Electrophoresis in the United States Pharmacopeia USP31 NF26 2nd Supplement, and chapter 2.2.31. Electrophoresis in the European Pharmacopoeia.
In the Ph. Eur. the harmonised chapter has been included as a section entitled Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), within a more general chapter entitled Electrophoresis. The general chapter includes other parts: General principle, Free or moving boundary electrophoresis, Zone electrophoresis using a supporting medium, and Polyacrylamide rod gel electrophoresis, which are not within the scope of pharmacopoeial harmonisation. The corresponding parts have been placed between black diamonds (♦♦).
The above differences in the Ph. Eur. text do not affect harmonisation as the general chapter provides additional information.
The texts of the 3 pharmacopoeias are therefore considered harmonised.
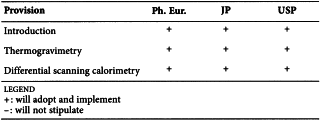
2.2.34. THERMAL ANALYSIS
As a result of the process of pharmacopoeial harmonisation, the following reflects the agreement reached by the Ph. Eur., the JP and the USP.
Harmonised provisions
Non-harmonised provisions
-
Local requirements
Thermogravimetry can be used as an alternative method for 2.41 Loss on Drying Test or 2.48 Water Determination (Karl Fischer Method). However, it must be confirmed beforehand that no volatile component except for water is included in the test specimen when thermogravimetry is used as an alternative method for water determination (Karl Fischer method). (JP)
(sign-off date: 26 June 2014)
2.2.47. Capillary electrophoresis
As a result of an evaluation of the texts 4. Capillary Electrophoresis in the Japanese Pharmacopoeia XV and <1053> Biotechnology-derived articles – Capillary Electrophoresis in the United States Pharmacopeia USP33 NF28, and chapter 2.2.47. Capillary electrophoresis in the European Pharmacopoeia, the texts of the 3 pharmacopoeias are considered harmonised.
2.2.54. Isoelectric focusing
As a result of an evaluation of the texts 9. Isoelectric Focusing in the Japanese Pharmacopoeia XV and <1054> Biotechnology-derived articles – Isoelectric Focusing in the United States Pharmacopeia USP33 NF28, and chapter 2.2.54. Isoelectric focusing in the European Pharmacopoeia, the texts of the 3 pharmacopoeias are considered harmonised.
2.2.55. Peptide mapping
The following comparative commentary refers to the texts 15. Peptide Mapping in the Japanese Pharmacopoeia XV and <1055> Biotechnology-derived Articles – Peptide Mapping in the United States Pharmacopeia USP31 NF26 2nd Supplement, and chapter 2.2.55. Peptide mapping in the European Pharmacopoeia.
Validation (USP)
The USP has entitled this part System Suitability. This terminology has been accepted by the 3 pharmacopoeias.
The use of peptide mapping for genetic stability evaluation (USP)
This additional section does not impact harmonisation since it is used only in development.
The above differences in the USP text do not affect harmonisation.
The texts of the 3 pharmacopoeias are therefore considered harmonised.
2.2.56. Amino acid analysis
The following comparative commentary refers to the texts 1. Amino Acid Analysis in the Japanese Pharmacopoeia XV and <1052> Biotechnology-derived Articles – Amino Acid Analysis in the United States Pharmacopeia USP31 NF26 1st Supplement, and chapter 2.2.56. Amino acid analysis in the European Pharmacopoeia.
Methodologies of amino acid analysis: general principles (USP)
The USP has replaced ‘6-aminoquinolyl-N-hydroxysuccinimidyl carbamate or o-phthalaldehyde’ with ‘6-aminoquinolyl-N-hydroxysuccinimidyl carbonate’.
These reagents are different but compatible and the use of one or the other does not affect harmonisation.
The USP has added a detailed example to describe each method listed below:
The above examples are given for further information and do not affect harmonisation.
The texts of the 3 pharmacopoeias are therefore considered harmonised.
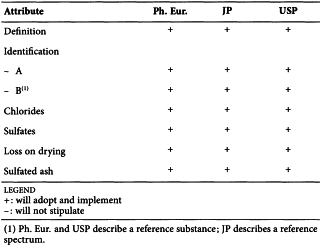
2.4.14. Sulfated ash
The following comparative commentary refers to the texts 2.44 Residue on Ignition Test in the Japanese Pharmacopoeia XV and <281> Residue on Ignition in the United States Pharmacopeia USP32 NF27 1st Supplement, and chapter 2.4.14. Sulfated ash in the European Pharmacopoeia.
The JP has added a non-harmonised introductory part, included between black diamonds, at the beginning of this chapter. It is given for further information and therefore does not affect harmonisation.
The USP text allows for the test to be performed at an ignition temperature other than 600 ± 50 °C if prescribed in an individual monograph. In the same way, a sample mass different from the usual quantity of 1-2 g can be used if prescribed in an individual monograph.
The USP has added a section, included between black diamonds, on the use of a muffle furnace and its calibration.
The above differences in the USP text do not affect harmonisation.
The texts of the 3 pharmacopoeias are therefore considered harmonised.
NOTE ICH has declared this method interchangeable within the ICH regions.
2.6.1. STERILITY
The following comparative commentary refers to the texts 4.06 Sterility Test in the partial revision of the Japanese Pharmacopoeia XV made official March 31, 2009, by the Ministry of Health, Labour and Welfare Ministerial Notification No. 190 and <71> Sterility Tests in the United States Pharmacopeia as presented in Pharmacopeial Forum, Volume 34(6), Interim Revision Announcement No. 6, December 1, 2008, official on May 1, 2009, and chapter 2.6.1. Sterility in the European Pharmacopoeia.
The USP has added requirements that cover either pharmacy bulk packages of antibiotics or medical devices. The corresponding parts, which are not within the scope of pharmacopoeial harmonisation, have been placed between black diamonds (♦♦).
The JP has deleted the requirements for ‘Catgut and other surgical sutures for veterinary use’ in Table 2, in the section ‘Direct inoculation of the culture medium’ and in Table 3. Catgut and other surgical sutures are outside the scope of the JP.
The above differences in the JP and USP texts do not affect harmonisation.
The texts of the 3 pharmacopoeias are therefore considered harmonised.
NOTE ICH has declared this method interchangeable within the ICH regions.
2.6.12. Microbiological examination of non-sterile products: microbial enumeration tests
As a result of an evaluation of the texts 4.05 Microbiological Examination of Non-sterile Products: I. Microbiological Examination of Non-sterile Products – Microbial Enumeration Tests in the Japanese Pharmacopoeia XV 1st Supplement and <61> Microbiological Examination of Non-sterile Products: Microbial Enumeration Tests in the United States Pharmacopeia USP30 NF25, and chapter 2.6.12. Microbiological examination of non-sterile products: microbial enumeration tests in the European Pharmacopoeia, the texts of the 3 pharmacopoeias are considered harmonised.
NOTE ICH has declared this method interchangeable within the ICH regions.
2.6.13. Microbiological examination of non-sterile products: test for specified micro-organisms
As a result of an evaluation of the texts 4.05 Microbiological Examination of Non-sterile Products: II. Microbiological Examination of Non-sterile Products – Test for Specified Micro-organisms in the Japanese Pharmacopoeia XV 1st Supplement and <62> Microbiological Examination of Non-sterile Products: Test for Specified Micro-organisms in the United States Pharmacopeia USP30 NF25, and chapter 2.6.13. Microbiological examination of non-sterile products: test for specified micro-organisms in the European Pharmacopoeia, the texts of the 3 pharmacopoeias are considered harmonised.
NOTE ICH has declared this method interchangeable within the ICH regions.
2.6.14. BACTERIAL ENDOTOXINS
As a result of an evaluation of the texts 4.01 Bacterial Endotoxin Test in the Japanese Pharmacopoeia XVI and <85> Bacterial Endotoxin Test in the United States Pharmacopoeia USP33, and chapter 2.6.14. Bacterial endotoxins in the European Pharmacopoeia, the texts of the 3 pharmacopoeias are considered harmonised.
NOTE ICH has declared these texts interchangeable within the ICH regions subject to the conditions detailed below.
1. Any of the 3 techniques can be used for the test. In the event of doubt or dispute, the gel-clot limit test should be used to make the final decision on compliance for the product being tested.
2. The USP, JP and Ph. Eur. reference standards are considered interchangeable as they have been suitably calibrated against the WHO (World Health Organization) International Standard for Endotoxin.
3. In the section Photometric quantitative techniques - Preparatory testing - Test for interfering factors, the user should perform the test on solutions A, B, C and D on at least 2 replicates using the optimal conditions as recommended by the lysate manufacturer.
2.9.1. DISINTEGRATION OF TABLETS AND CAPSULES
The following comparative commentary refers to the texts 6.09 Disintegration Test in the Japanese Pharmacopoeia XV and <701> Disintegration in the United States Pharmacopeia USP32 NF27 1st Supplement, and chapter 2.9.1. Disintegration of tablets and capsules (Test A) in the European Pharmacopoeia.
In the Ph. Eur. chapter, test A corresponds to the harmonised chapter while test B does not and is intended for tablets and capsules that are greater than 18 mm long. Test B is not within the scope of pharmacopoeial harmonisation and has been placed between black diamonds (♦♦).
The JP and USP specify procedures and acceptance criteria for different types of dosage forms. The equivalent statements are included in the Ph. Eur. general monographs on dosage forms. These statements are not within the scope of pharmacopoeial harmonisation.
In addition, the JP describes an auxiliary tube, and a metal plate to secure the glass tubes. This has been placed between black diamonds (♦♦). The use of this tube and this plate may have an impact on hydrodynamics and thus may affect harmonisation.
The texts of the 3 pharmacopoeias are therefore considered harmonised.
NOTE ICH has declared this method interchangeable within the ICH regions subject to the conditions detailed below.
For tablets and capsules larger than 18 mm long, for which a different apparatus is used, the disintegration test is not considered to be interchangeable in the 3 regions.
The test for disintegration is not considered to be interchangeable in the 3 regions for dosage forms referred to in the pharmacopoeias as delayed-release, gastro-resistant or enteric-coated.
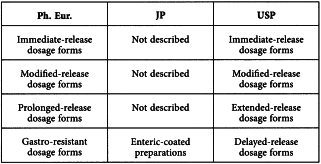
2.9.3. DISSOLUTION TEST FOR SOLID DOSAGE FORMS
As a result of the process of pharmacopoeial harmonisation, the following reflects the agreement reached by the Ph. Eur., the JP and the USP.
Harmonised provisions
Local requirements
Local requirements have been placed between white diamonds (♢♢) in Ph. Eur. chapter.
The terminology used to describe the dosage forms has not been harmonised:
NOTE ICH has declared this method interchangeable within the ICH regions subject to the conditions detailed below.
(Sign-off date: 10 June 2010)
2.9.7. Friability of uncoated tablets
As a result of an evaluation of the texts 26. Tablet Friability Test in the Japanese Pharmacopoeia XV and <1216> Tablet Friability in the United States Pharmacopeia USP31 NF26 1st Supplement, and chapter 2.9.7. Friability of uncoated tablets in the European Pharmacopoeia, the texts of the 3 pharmacopoeias are considered harmonised.
2.9.17. Test for extractable volume of parenteral preparations
The following comparative commentary refers to the texts 6.05 Test for Extractable Volume of Parenteral Preparations in the Japanese Pharmacopoeia XV and <1> Injections in the United States Pharmacopeia USP32 NF27 1st Supplement, and chapter 2.9.17. Test for extractable volume of parenteral preparations in the European Pharmacopoeia.
The JP has added a non-harmonised introductory part, included between black diamonds, at the beginning of this chapter. It is given for further information and does not affect harmonisation.
The USP has included this test in general chapter <1> Injections, under a specific part entitled Determination of Volume of Injection in Containers. This does not affect harmonisation.
The texts of the 3 pharmacopoeias are therefore considered harmonised.
NOTE ICH has declared this method interchangeable within the ICH regions.
2.9.19. PARTICULATE CONTAMINATION: SUB-VISIBLE PARTICLES
The following comparative commentary refers to the texts 6.07 Insoluble Particulate Matter Test for Injections in the Japanese Pharmacopoeia XV (corrected version dated September 2007) and <788> Particulate Matter in Injections in the United States Pharmacopeia USP32 NF27 2nd Supplement, and chapter 2.9.19. Particulate contamination: sub-visible particles in the European Pharmacopoeia.
The USP specifies that system suitability can be verified using USP Particle Count RS. This statement is not within the scope of pharmacopoeial harmonisation. It has been placed between black diamonds (♦♦) and it does not affect harmonisation.
The JP includes a detailed section on calibration of the apparatus. In particular, requirements for the quality of particle-free water are given, which differ from those stated in the USP (see section Reagents, Indicators and Solutions) and in the Ph. Eur. (see chapter 4.1.1). The section on calibration is not within the scope of pharmacopoeial harmonisation. It has been placed between black diamonds (♦♦) and it does not affect harmonisation.
In addition, the JP describes more stringent acceptance criteria for parenteral preparations having a nominal volume of 100 mL. This was acknowledged by the PDG as a non-harmonised item. It has been placed between black diamonds (♦♦). The acceptance criteria for parenteral preparations having a nominal volume of 100 mL are therefore considered non-harmonised.
The texts of the 3 pharmacopoeias are therefore considered harmonised except for the acceptance criteria for parenteral preparations having a nominal volume of 100 mL.
NOTE ICH has declared this method interchangeable within the ICH regions except the acceptance criteria for parenteral preparations having a nominal volume of 100 mL.
2.9.26. Specific surface area by gas adsorption
The following comparative commentary refers to the texts 3.02 Specific Surface Area by Gas Adsorption in the Japanese Pharmacopoeia XV and <846> Specific Surface Area in the United States Pharmacopeia USP31 NF26 1st Supplement, and chapter 2.9.26. Specific surface area by gas adsorption in the European Pharmacopoeia.
The JP has chosen to express all the temperatures of this chapter in degrees Celsius.
Multi-point measurement (JP)
The JP does not state the meaning of the 22400 constant in the definition of the specific surface area S and does not require a test to determine the linearity of the method.
Single-point measurement (JP)
The JP does not state the equivalent quantity of gas corresponding to the value of P/P0, which is less precise (0.30) than in the other pharmacopoeias (0.300).
The JP does not assume the material constant C to be invariant.
Measurements (JP)
The JP does not specify the temperature required to perform the test for either method.
The JP limits its volumetric method to classical instruments and does not take alternative instruments into account.
The above differences in the JP text might affect harmonisation.
Therefore only the texts of the Ph. Eur. and the USP are considered harmonised.
2.9.36. Powder flow
The following comparative commentary refers to the texts 18. Powder Flow in the Japanese Pharmacopoeia XV and <1174> Powder Flow in the United States Pharmacopeia USP31 NF26 1st Supplement, and chapter 2.9.36. Powder flow in the European Pharmacopoeia.
Flow through an orifice (JP)
The JP limits the use of orifices to classical ones and does not allow vibrators or moving orifices. A test result using the JP method will be compatible with the Ph. Eur. and the USP. A Ph. Eur. or USP test result will not comply with the JP when a vibrator or moving orifice is used.
2.9.37. Optical microscopy
As a result of an evaluation of the texts 3.04 Particle Size Determination in the Japanese Pharmacopoeia XV and <776> Optical Microscopy in the United States Pharmacopeia USP31 NF26 2nd Supplement, and chapter 2.9.37. Optical microscopy in the European Pharmacopoeia, the texts of the 3 pharmacopoeias are considered harmonised.
2.9.38. Particle-size distribution estimation by analytical sieving
The following comparative commentary refers to the texts 3.04 Particle Size Determination in the Japanese Pharmacopoeia XV and <786> Particle-size Distribution Determination by Analytical Sieving in the United States Pharmacopeia USP31 NF26 1st Supplement, and chapter 2.9.38. Particle-size distribution estimation by analytical sieving in the European Pharmacopoeia.
Sieving methods - Dry sieving method (JP)
The JP permits any powder on the down surface of the sieve to be brushed and combined with the fraction of the next sieve.
The above difference in the JP text might affect harmonisation.
Therefore only the texts of the Ph. Eur. and the USP are considered harmonised.
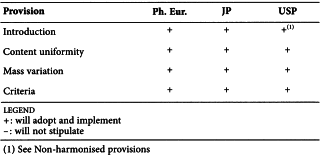
2.9.40. UNIFORMITY OF DOSAGE UNITS
As a result of the process of pharmacopoeial harmonisation, the following reflects the agreement reached by the Ph. Eur., the JP and the USP.
Harmonised provisions
Non-harmonised provisions
The following statement in the introduction is not accepted and will not be included by the USP.
“Alternatively, products listed in item (4) above that do not meet the 25 mg/25% threshold limit may be tested for uniformity of dosage units by Mass Variation instead of the Content Uniformity test if the concentration relative standard deviation (RSD) of the drug substance in the final dosage units is not more than 2%, based on process validation data and development data, and if there has been regulatory approval of such a change. The concentration RSD is the RSD of the concentration per dosage unit (w/w or w/v), where concentration per dosage unit equals the assay result per dosage unit divided by the individual dosage unit weight. See the RSD formula in Table 2.”
The corresponding statement in the Ph. Eur. has been placed between black diamonds (♦♦).
Local requirements
The Ph. Eur. prescribes in its relevant general monographs on dosage forms that preparations supplied in single-dose containers that represent 1 dose of medicinal product and are intended for transdermal delivery of the active substance(s) in view of a systemic effect comply with general chapter 2.9.40. Uniformity of dosage units. Therefore the wording has been adapted as follows:
“Unless otherwise stated, the uniformity of dosage units specification is not intended to apply to solutions, suspensions, emulsions or gels in single-dose containers intended for local action following cutaneous administration.”
The Ph. Eur. excludes multivitamin, single-vitamin and trace-element preparations from the test for content uniformity.
In the Ph. Eur. section on mass variation the procedure for liquid dosage forms also applies to semi-solid dosage forms.
All local requirements have been placed between white diamonds (♢♢) in Ph. Eur. chapter.
NOTE ICH has declared this method interchangeable within the ICH regions subject to the conditions detailed below.
(sign-off date: 4 November 2015)
5.1.4. Microbiological quality of non-sterile pharmaceutical preparations and substances for pharmaceutical use
The following comparative commentary refers to the texts 12. Microbial Attributes of Non-sterile Pharmaceutical Products in the Japanese Pharmacopoeia XV 1st Supplement and <1111> Microbiological Attributes of Non-sterile Pharmaceutical Products in the United States Pharmacopeia USP30 NF25, and chapter 5.1.4. Microbiological quality of non-sterile pharmaceutical preparations and substances for pharmaceutical use in the European Pharmacopoeia.
Two special Ph. Eur. provisions are included within Table 5.1.4.-1: for oral dosage forms containing raw materials of natural origin and for premixes for medicated feeding stuffs for veterinary use. Also, a reference to chapter 5.1.8 giving recommended acceptance criteria for the microbiological quality of herbal medicinal products for oral use and extracts used in their preparation is included in the text. The corresponding parts, which are not within the scope of pharmacopoeial harmonisation, have been placed between black diamonds (♦♦).
The above differences in the Ph. Eur. text do not affect harmonisation.
The texts of the 3 pharmacopoeias are therefore considered harmonised.
NOTE ICH has declared these texts interchangeable within the ICH regions.
Monographs
As a result of the process of pharmacopoeial harmonisation, the following reflects the agreement reached by the Ph. Eur., the JP and the USP.
Reagents and reference materials
Each pharmacopoeia adapts the text to take account of local reference materials and reagent specifications.
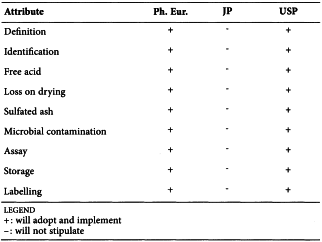
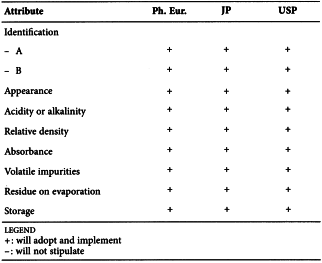
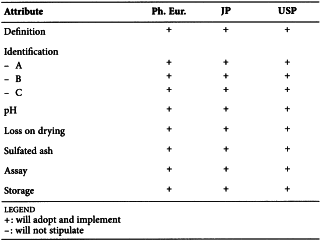
CARMELLOSE (2360)
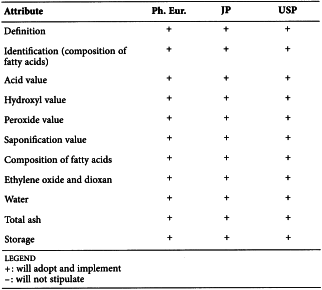
Harmonised attributes
Non-harmonised attributes
Characters/Description, Heavy metals, Storage/Containers and storage/Packaging and storage
Local requirements
-
(sign-off date: 9 November 2011)
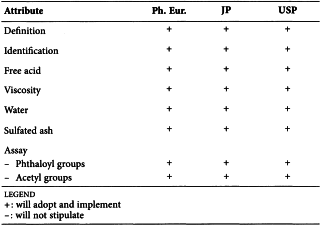
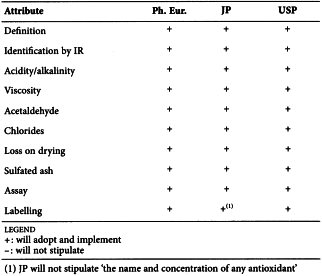
CELLULOSE ACETATE (0887)
Harmonised attributes
JP will not include this monograph and has therefore not participated in the harmonisation.
Non-harmonised attributes
-
Local requirements
Characters ( Ph. Eur.)
(sign-off date: 26 May 2016)
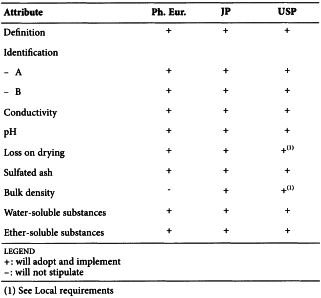
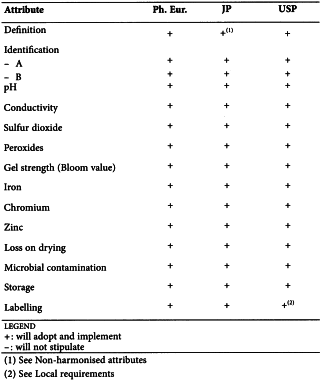
CELLULOSE ACETATE PHTHALATE (0314)
Harmonised attributes
Non-harmonised attributes
Characters/Description and solubility/Description, Heavy metals, Packaging and storage
Local requirements
-
(sign-off date: 9 November 2010)
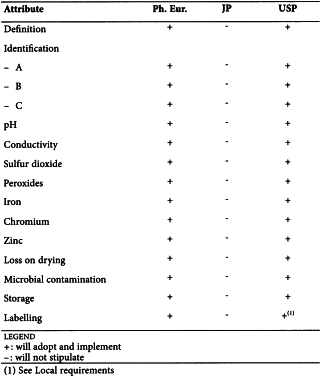
CELLULOSE, MICROCRYSTALLINE (0316)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Microbial contamination, Labelling, Containers and storage/Packaging and storage
Local requirements
Solubility ( Ph. Eur.), Loss on drying (the value can be within a percentage range, as specified on the labelling) (USP), Particle size distribution estimation by analytical sieving (USP), Definition (reference to labelling) (JP), Identification (2) (dispersion test) (JP), Loss on drying (the value can be within a percentage range, as specified on the labelling) (JP), Heavy metals (JP), Sulfated ash (on 2.0 g) (JP)
(sign-off date: 26 May 2016)
Cellulose, powdered (0315)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Microbial contamination, Containers and storage/Packaging and storage, Labelling
Local requirements
Solubility ( Ph. Eur.), Definition (after partial hydrolysis as occasion demands) (JP), Identification (2) (dispersion test) (JP), Heavy metals (JP)
(sign-off date: 26 May 2016)
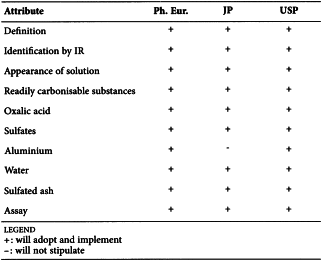
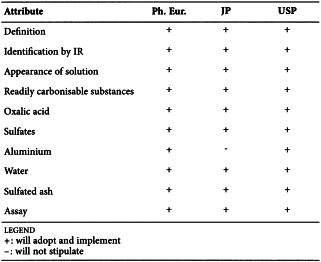
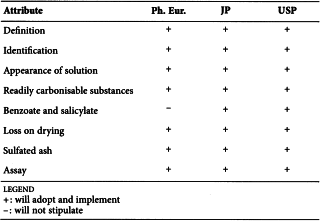
CITRIC ACID (0455)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Heavy metals, Bacterial endotoxins, Labelling, Storage
Local requirements
Identification E (water) ( Ph. Eur.), Second identification (identifications A, C, D, E) ( Ph. Eur.), Sterility (USP)
(sign-off date: 9 June 2010)
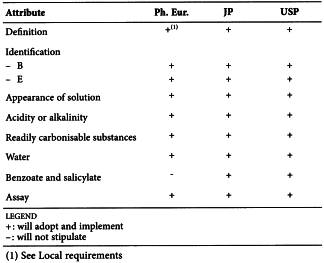
CITRIC ACID MONOHYDRATE (0456)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Heavy metals, Bacterial endotoxins, Labelling, Storage
Local requirements
Identification E (water) ( Ph. Eur.), Second identification (identifications A, C, D, E) ( Ph. Eur.), Sterility (USP)
(sign-off date: 9 June 2010)
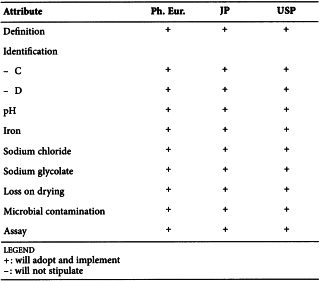
CROSCARMELLOSE SODIUM (0985)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Sodium chloride and sodium glycolate, Water-soluble substances, Microbial contamination, Packaging and storage/Containers and storage
Local requirements
Identification of sodium using potassium pyroantimonate ( Ph. Eur.), Heavy metals (JP)
(sign-off date: 26 May 2016)
CROSPOVIDONE (0892)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Identification by IR, Heavy metals, Labelling.
Local requirements
-
(sign-off date: 9 November 2010)
ETHANOL (96 PER CENT) (1317)
Harmonised attributes
Definition and Relative density
Each pharmacopoeia specifies a different range for the content; the values for relative density vary accordingly and, in addition, are expressed at different temperatures.
Non-harmonised attributes
Characters/Description
Local requirements
Identification tests C and D ( Ph. Eur.), List of impurities (Ph. Eur.), Expiration date (JP)
(sign-off date: 7 November 2012)
ETHANOL, ANHYDROUS (1318)
Harmonised attributes
Definition and Relative density
Each pharmacopoeia specifies a different range for the content; the values for relative density vary accordingly and, in addition, are expressed at different temperatures.
Non-harmonised attributes
Characters/Description
Local requirements
Identification tests C and D ( Ph. Eur.), List of impurities (Ph. Eur.), Expiration date (JP)
(sign-off date: 7 November 2012)
ETHYLCELLULOSE (0822)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Containers and storage/Packaging and storage
Local requirements
Identification (compliance with limits of assay) (Ph. Eur.), Heavy metals (JP)
(sign-off date: 26 May 2016)
GELATIN (0330)
Gelling grade
Harmonised attributes
Non-harmonised attributes
Definition (JP) ‘and/or enzymatic hydrolysis’ not included
Characters/Description
Local requirements
Heavy metals (JP), Arsenic (JP), Containers (JP), Labelling (method of hydrolysis) (USP)
(sign-off date: 26 October 2016)
Non-gelling grade
Harmonised attributes
JP has signed the draft for non-gelling grade but will not implement it as this grade is hardly ever used in Japan.
Non-harmonised attributes
Characters
Local requirements
Labelling (method of hydrolysis) (USP)
(sign-off date: 26 October 2016)
GLUCOSE (0177)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Heavy metals, Containers and storage/Packaging and storage, Labelling
Local requirements
First identification (specific optical rotation) ( Ph. Eur.), Second identification (TLC, colour reaction) ( Ph. Eur.), Pyrogens ( Ph. Eur.), Identification (colour reaction) (JP), Specific optical rotation (JP), Identification (infrared absorption spectrophotometry) (USP)
(sign-off date: 4 November 2015)
GLUCOSE MONOHYDRATE (0178)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Heavy metals, Containers and storage/Packaging and storage, Labelling
Local requirements
First identification (specific optical rotation) ( Ph. Eur.), Second identification (TLC, colour reaction) ( Ph. Eur.), Pyrogens ( Ph. Eur.), Identification (colour reaction) (JP), Specific optical rotation (JP), Identification (infrared absorption spectrophotometry) (USP)
(sign-off date: 4 November 2015)
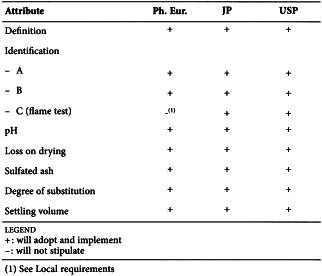
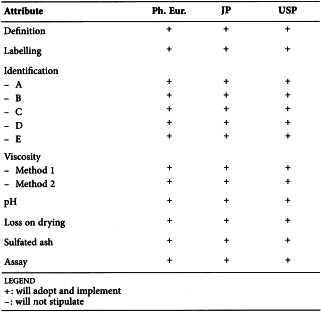
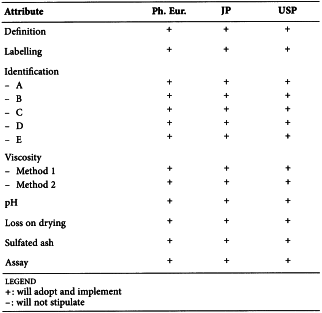
HYDROXYETHYLCELLULOSE (0336)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Viscosity, Labelling, Containers and storage/Packaging and storage
Local requirements
Second identification (identifications B, C, D, E) ( Ph. Eur.), Ethylene oxide ( Ph. Eur.), 2-Chloroethanol ( Ph. Eur.), Heavy metals (JP), Lead (USP)
(sign-off date: 25 July 2016)
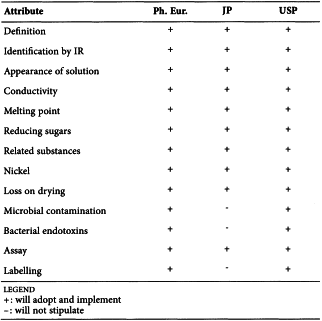
HYDROXYPROPYLCELLULOSE (0337)
Harmonised attributes
Non-harmonised attributes
Characters/Description/Description and solubility, Packaging and storage, Heavy metals, Viscosity, Labelling
Local requirements
Identification (cloud point) ( Ph. Eur.), Lead (USP)
(sign-off date: 27 June 2013)
HYDROXYPROPYLCELLULOSE, LOW-SUBSTITUTED (2083)
Harmonised attributes
Non-harmonised attributes
Characters/Description
Local requirements
Chloride and sulfate (USP)
(sign-off date: 26 October 2016)
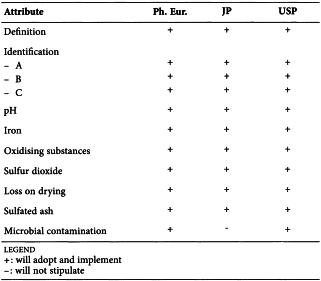
HYPROMELLOSE (0348)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Containers and storage/Packaging and storage
Local requirements
Appearance of solution ( Ph. Eur.), Heavy metals (JP)
(sign-off date: 26 October 2016)
ISOMALT (1531)
Harmonised attributes
Non-harmonised attributes
Characters/Description (including test for optical rotation), Heavy metals, Packaging and storage
Local requirements
Second identification (TLC, colour reaction) ( Ph. Eur.), Identification (colour reaction) (JP), Identification/assay (Reference solution (a). Dissolve 0.2 g of isomalt RS in 4 mL of water and dilute to 10.0 mL with the same solvent) (JP), Identification by TLC (USP)
(sign-off date: 4 November 2015)
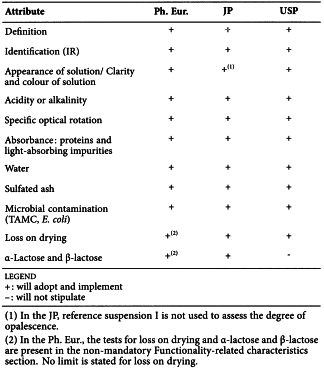
LACTOSE (1061)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Labelling, Containers and storage/Packaging and storage
Local requirements
Identification D ( Ph. Eur.), Microbial contamination (TYMC, Salmonella) (JP), Heavy metals (JP), Identification B (USP), Microbial contamination (TYMC) (USP), Content of alpha and beta anomers (a limit is to be stated on the label where necessary) (USP)
(sign-off date: 26 October 2016)
LACTOSE MONOHYDRATE (0187)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Microbial contamination, Heavy metals, Labelling, Containers and storage/Packaging and storage
Local requirements
Identification B and C (USP), Particle-size distribution (USP), Loss on drying (USP)
(sign-off date: 5 June 2008)
MAGNESIUM STEARATE (0229)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Microbial contamination, Specific surface area, Labelling, Packaging and storage
Local requirements
Second identification (identifications A, B, D) ( Ph. Eur.)
(sign-off date: 27 June 2013)
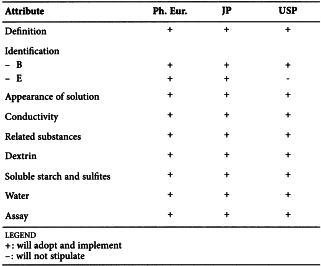
MAIZE STARCH (0344)
Harmonised attributes
Non-harmonised attributes
Characters, Containers and storage/Packaging and storage
Local requirements
Foreign Matter (Ph. Eur., JP), Absence of Salmonella (Ph. Eur.), Absence of Staphylococcus aureus and Pseudomonas aeruginosa when the product is intended for use in preparing Absorbable Dusting Powder (USP), Labelling (USP)
(sign-off date: 6 June 2012)
MANNITOL (0559)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Heavy metals, Container and storage/Packaging and storage
Local requirements
Second identification (specific optical rotation, melting point, TLC) ( Ph. Eur.), Absence of Salmonella ( Ph. Eur.)
(sign-off date: 6 June 2012)
METHYLCELLULOSE (0345)
Harmonised attributes
Non-harmonised attributes
Characters, Heavy metals, Packaging and storage
Local requirements
Appearance of solution ( Ph. Eur.), Description (JP)
(sign-off date: 26 June 2014)
POLYSORBATE 80 (0428)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Identification by IR
Local requirements
Second identification (identifications B, C, E) ( Ph. Eur.), Heavy metals (JP)
(sign-off date: 26 October 2016)
POTATO STARCH (0355)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Containers and storage/Packaging and storage
Local requirements
Foreign matter ( Ph. Eur.), Absence of Salmonella ( Ph. Eur.)
(sign-off date: 15 June 2011)
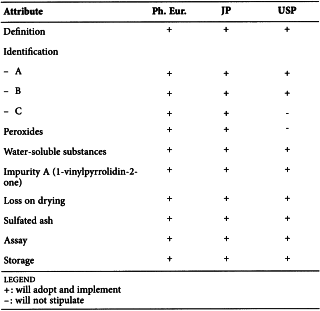
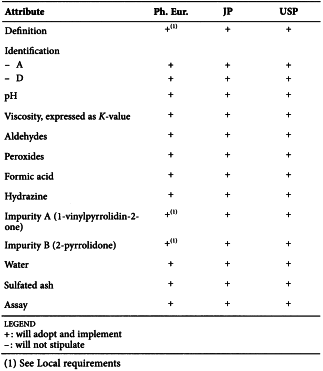
POVIDONE (0685)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Appearance of solution, Storage/Containers and storage/Packaging and storage
Local requirements
Definition (chemical formula and chemical structure) ( Ph. Eur.), Second identification (identifications B, C, D) ( Ph. Eur.), Viscosity, expressed as K-value (size no. 1 viscometer, minimum flow time 100 s) ( Ph. Eur.), Impurity A (retention time of vinyl acetate and relative retention of impurity A, washing of precolumn by mobile phase back flow) ( Ph. Eur.), Impurity B (washing of precolumn by mobile phase back flow) ( Ph. Eur.), Heavy metals (JP), Identification B (dichromate test) (USP), Identification C (thiocyanate test) (USP), Identification D (iodine test) (USP)
(sign-off date: 4 November 2015)
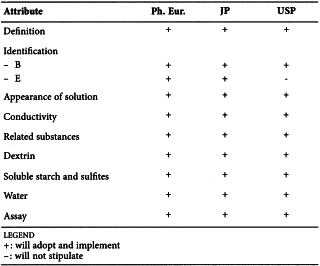
RICE STARCH (0349)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Foreign matter, Containers and storage/Packaging and storage
Local requirements
Absence of Salmonella ( Ph. Eur.)
(sign-off date: 6 November 2013)
SACCHARIN (0947)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Melting point, Packaging and storage, Toluenesulfonamides, Heavy metals
Local requirements
Second identification (identifications A, B, D, E) ( Ph. Eur.)
(sign-off date: 27 June 2013)
SACCHARIN SODIUM (0787)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Toluenesulfonamide, Storage/Containers and storage/Packaging and storage, Labelling
Local requirements
Definition (‘It may be anhydrous or contain a variable quantity of water’) ( Ph. Eur.), Second identification (identifications A, C, D, E) ( Ph. Eur.), Heavy metals (JP)
(sign-off date: 26 October 2016)
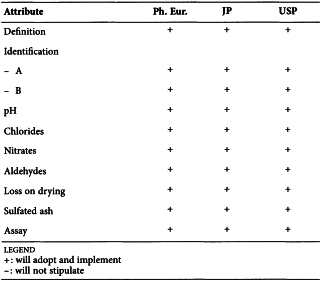
SODIUM CHLORIDE (0193)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Appearance of solution, Arsenic, Heavy metals, Bacterial endotoxins, Labelling, Sterility, Storage
Local requirements
-
(sign-off date: 26 May 2016)
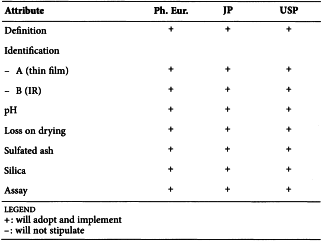
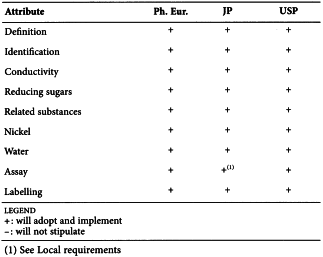
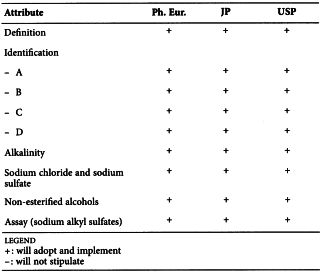
SODIUM LAURILSULFATE (0098)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Storage/Containers and storage/Packaging and storage
Local requirements
Water (JP), Total alcohols (JP, USP)
(sign-off date: 13 November 2014)
SODIUM STARCH GLYCOLATE (TYPE A) (0983)SODIUM STARCH GLYCOLATE (TYPE B) (0984)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Identification (IR), Heavy metals, Labelling, Packaging and storage
Local requirements
Appearance of solution ( Ph. Eur.), Identification (complies with pH test) ( Ph. Eur.), Identification (formation of a suspension) ( Ph. Eur.), Identification (sodium flame test) (USP)
(sign-off date: 27 June 2013)
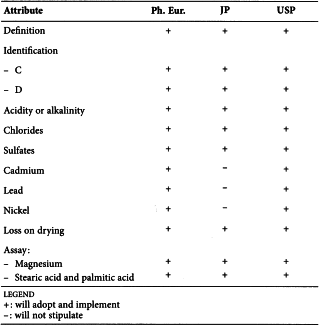
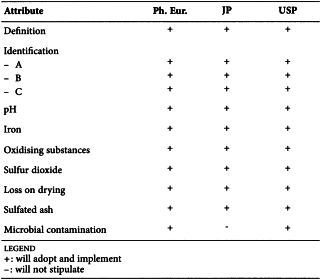
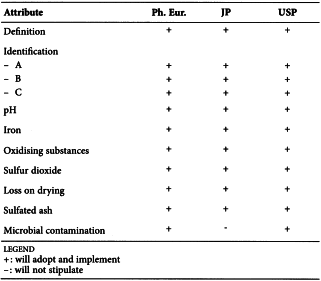
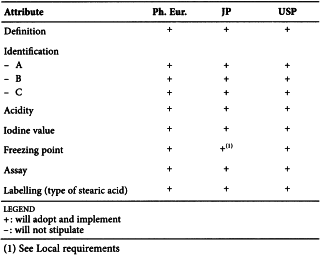
STEARIC ACID (1474)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Nickel, Labelling (source of stearic acid)
Local requirements
Appearance ( Ph. Eur.), Heavy metals (JP), Sulfated ash (JP), Containers and storage (JP), Freezing point (alternative apparatus) (JP), Microbial contamination (USP)
(sign-off date: 1st July 2015)
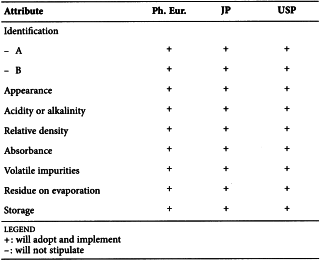
SUCROSE (0204)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Identification, Colour value, Packaging and storage
Local requirements
-
(sign-off date: 27 June 2013)
WHEAT STARCH (0359)
Harmonised attributes
Non-harmonised attributes
Characters/Description, Packaging and storage/Containers and storage
Local requirements
Foreign matter ( Ph. Eur.), Absence of Salmonella ( Ph. Eur.), Total protein (catalyst: TiO2 instead of Se) (JP)
(sign-off date: 4 November 2015)