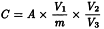Appendix VIII W. Determination of Elemental Impurities
Introduction
This chapter describes the general approach for the determination of elemental impurities in medicinal products or substances for pharmaceutical use. As the chemical composition of the considered samples and the specification limits for the element(s) of interest vary considerably, it is not possible to describe all suitable sample preparation and measurement methods. Therefore, any method that fulfils the requirements described in this chapter may be used.
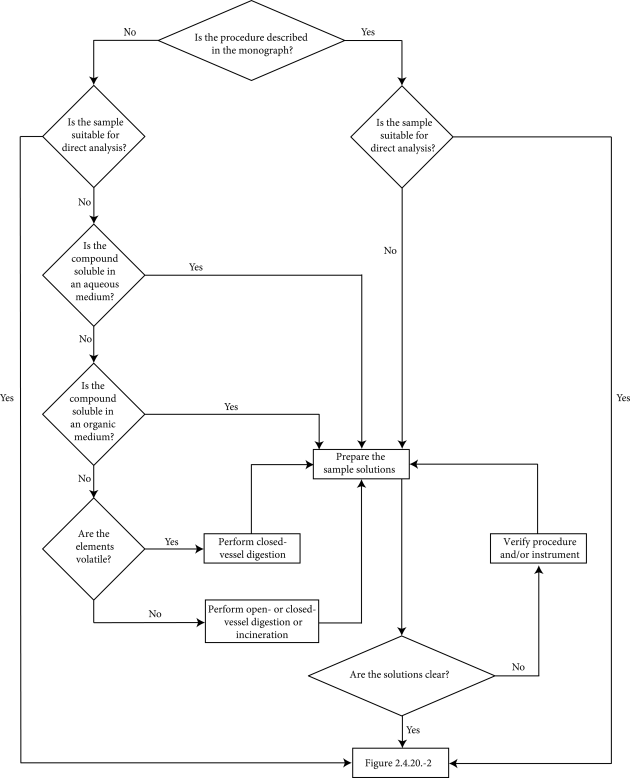
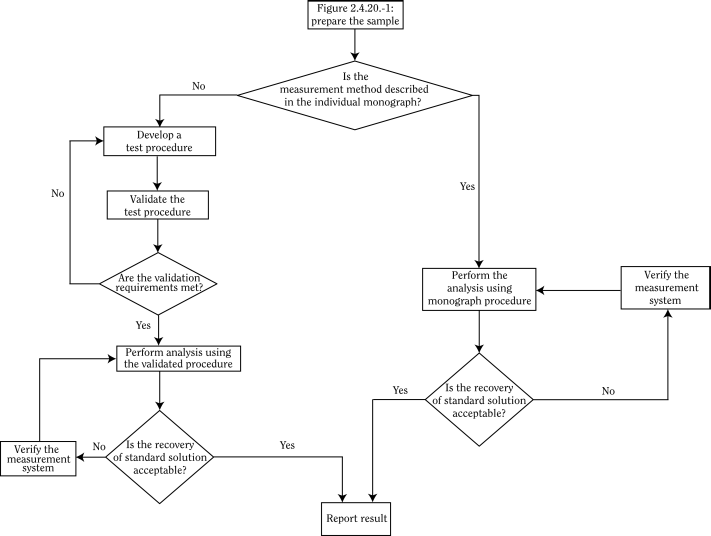
The results of the analysis are acceptable only if the system suitability has been demonstrated by a suitable test. Before the initial use of a method, the analyst must ensure that the method is appropriate for the samples and instruments used. This is accomplished by applying a validation procedure to methods not described in the individual monograph or by a system suitability test for methods which are described in the monograph. Decision trees for the choice of the sample preparation and the measurement procedures are presented in Figures 2.4.20.-1 and 2.4.20.-2.
Procedures
As a reference procedure is not provided for each element, matrix and concentration, the choice of procedure according to Figures 2.4.20.-1 and 2.4.20.-2, including sample preparation, detection technique and instrument parameters, is the responsibility of the user.
Use the flow chart in Figure 2.4.20.-1 to define the sample preparation method and the flow chart in Figure 2.4.20.-2 to define the measurement method. The sample preparation method should yield a sufficient quantity of sample to allow quantification of each element at the specified limit stated in the individual monograph or the general chapter.
All suitable sample preparation methods and measurement techniques (e.g. 2.2.22. Atomic emission spectrometry (AES), 2.2.23. Atomic absorption spectrometry (AAS), 2.2.37. X-ray fluorescence spectrometry (XRFS), 2.2.57. Inductively coupled plasma-atomic emission spectrometry (ICP-AES), 2.2.58. Inductively coupled plasma-mass spectrometry (ICP-MS), 2.4.2. Arsenic, 2.4.8. Heavy metals, 2.4.9. Iron, 2.4.10. Lead in sugars, 2.4.15. Nickel in polyols, 2.4.31. Nickel in hydrogenated vegetable oils) can be used for the determination of elemental impurities, if the method has been verified before the initial use by a system suitability test or a validation procedure according to this chapter.
If no sample preparation and/or measurement method is described in the individual monograph, a suitable sample preparation and/or measurement method must be developed and validated (see Figures 2.4.20.-1 and 2.4.20.-2).
Sample preparation
Sample preparation is critical to the success of elemental analysis. Many techniques not using direct measurement are heavily dependent on sample transport.
If an atomisation system is used, the most conventional means by which samples are introduced into the atomisation system is by solution nebulisation. In this case, solid samples must be dissolved in order to be introduced into the atomisation system. Samples may be dissolved in any appropriate solvent. The use of aqueous or dilute nitric acid solutions is strongly recommended, due to minimal interference with these solvents compared to other solvents. Hydrochloric acid, hydrofluoric acid, perchloric acid, sulfuric acid and hydrogen peroxide, at various concentrations, can be used to dissolve the samples. The viscosity of sulfuric acid is greater than that of the other acids and is to be taken into account as it can affect the overall fluidity of the solution.
The choice of solvents also includes, but is not limited to, the use of dilute bases, straight or diluted organic solvents, combinations of acids or bases, and combinations of organic solvents.
Acids, bases, and hydrogen peroxide of high purity must be used, especially when ICP-MS is employed. For aqueous solutions, use deionised distilled water R. Diluents must be checked for interference if they are used in an analysis. Because it is not always possible to obtain organic solvents that are free from elemental impurities, organic solvents of the highest purity possible with regard to these contaminants must be used. Specifically for ICP techniques, where samples are introduced into the plasma via solution nebulisation, it is important to consider the potential matrix effects and interferences that might arise from the solvent. The use of an appropriate internal standard and/or matching the standard matrix with samples should be applied for ICP-AES and ICP-MS analyses in cases where accuracy and precision are not sufficient. In any case, the selection of an appropriate internal standard should take into account the element(s) of interest, ionisation energy, wavelengths or masses, and the nature of the sample matrix.
Where a sample is found not to be soluble in any acceptable solvent, a variety of digestion or incineration techniques can be employed. These include hot-plate digestion, incineration and microwave-assisted digestions, using an open- or closed-vessel.
The decision regarding the type of digestion technique to be used depends on the nature of the sample being digested, as well as on the element(s) of interest and the concentration range of the elements to be quantified. Open-vessel digestion is not recommended for the analysis of volatile elements. The suitability of a digestion technique, whether open- or closed-vessel, should be supported by spike recovery experiments in order to verify that, within an acceptable tolerance, volatile elements have not been lost during sample preparation. The digestion cycle is suitable if a clear solution is obtained.
It is important to consider the selection of the type, the material of construction, the pretreatment, and the cleaning of analytical labware used in elemental analyses. The material must be inert and, depending on the specific application, resistant to caustics, acids, and/or organic solvents. For some analyses, care must be exercised to prevent the adsorption of elemental impurities onto the surface of a vessel, particularly in ultra-trace analyses. Contamination of sample solutions by elemental impurities and ions present in the container can also lead to inaccurate results.
The use of volumetric glassware that does not comply with Class A requirements of the appropriate International Standard of the International Organization for Standardization (ISO) is acceptable if the validation or the system suitability test of the method using such glassware have experimentally demonstrated that the method is suitable for the intended purpose.
CAUTION: when using high-pressure digestion vessels and microwave laboratory equipment, the safety precautions and operating instructions given by the manufacturer must be followed.
Measurement
Method
The choice of the techniques depends mainly on the sample matrix and the characteristics and specification limits of the element(s) of interest. Analyse according to the instructions of the manufacturer of the equipment regarding program and wavelength.
System suitability
A system suitability test must be carried out on the day of the analysis to ensure that the sample preparation and measurement system are appropriate.
Acceptance criterion for preparation of sample solution A clear solution is obtained.
Acceptance criterion for measurement systemThe measured concentration of a standard solution of the element at a concentration within the range of the used calibration curve does not differ from the actual concentration by more than 20 per cent.
Calculation
The blank value of reagents must be taken into account for the calculation of the content. Upon completion of the analysis, the concentration of a given element in the sample is calculated by the software of the instrument from the concentration of the element in the test solution. If no calculation software is available or no indication for calculation is given in the general chapter corresponding to the method used, the concentration of a given element in the sample can be calculated from the concentration of the element in the solution using the following expression:
C | = | concentration of element in the analysed sample, in micrograms per gram; |
A | = | instrument reading of the concentration of the element in the sample solution, in micrograms per millilitre; |
m | = | mass of the sample in the initial sample solution, in grams; |
V1 | = | volume of the initial sample preparation, in millilitres; |
V2 | = | total volume of any dilution performed, in millilitres; |
V3 | = | volume of initial sample preparation used in any dilution performed, in millilitres. |
validation requirements
Some validation requirements provided below may differ from those provided in general chapters of the Ph. Eur. (e.g. 2.2.22 (AES), 2.2.23 (AAS), 2.2.57 (ICP-AES), 2.2.58 (ICP-MS)).
Before the initial use of the selected procedure, the analyst must ensure that the sample preparation and measurement method are appropriate for the element(s) of interest, sample matrix and instrument used. This is accomplished by following the validation procedure before the initial use and the system suitability test on the day of the analysis.
For elemental impurities, validation of a limit test must include specificity and limit of detection.
The following section defines the characteristics for the acceptability of a quantitative procedure. It must be demonstrated experimentally that such a procedure complies with the validation requirements, with an appropriate system suitability test using material spiked with a suitable reference material. The test materials must be spiked before any sample preparation steps. For example, if a test material is to be digested, the material must be spiked at the beginning of the digestion procedure.
Specificity
Specificity is the ability to ensure that the analytical procedure (sample preparation and measurement) allows a reliable determination of the element(s) of interest in the presence of components (e.g. carrier gas, impurities, matrix) that may be expected to be present.
Acceptance criteria The procedure must be able to assess unequivocally each elemental impurity to be determined with this procedure in the presence of components that may be expected to be present, including other elemental impurities, matrix components, and other sources of interference; specificity is demonstrated by complying with the accuracy requirement for the element(s) to be determined.
RANGE
Acceptance criterion range is demonstrated by complying with the recovery requirement.
Accuracy
Verify the accuracy using a certified reference material (CRM) or by performing a test for recovery.
The recovery may be determined on a sample of the substance to be examined, spiked with a known quantity of a reference standard of the element of interest (3 concentration levels in the range of 50-150 per cent of the intended specification limit, even if the original concentration of the reference standard is at the specified value), in triplicate.
Acceptance criterion Spike recovery is within 70 per cent and 150 per cent for the mean of 3 replicates at each concentration.
REPEATABILITY
Test samples either 6 independent samples of the substance to be examined spiked with a suitable reference standard at the specified concentration level, or 3 concentration levels prepared in triplicate.
Acceptance criterion The relative standard deviation is in both cases not more than 20 per cent.
Intermediate precision
The effect of random events (intra-laboratory variations) on the analytical precision of the method must be established. Acceptable experiments for establishing intermediate precision include performing the repeatability analysis on different days, or with different instrumentation, or by different analysts. Only 1 of the 3 experiments is required to demonstrate intermediate precision.
Acceptance criterion The relative standard deviation is not more than 25 per cent.
Limit of quantification
Use the results from the accuracy study. Determine the lowest concentration meeting the acceptance criterion.
Acceptance criterion The limit of quantification is below the specification limit.
LIMIT OF DETECTION (ONLY APPLICABLE TO LIMIT TESTS)
Determine the lowest concentration giving a signal clearly distinct from that obtained with a blank solution.
Acceptance criterion The limit of detection is not more than 0.5 times the concentration of the specification limit.