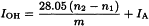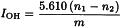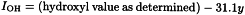Appendix X D. Hydroxyl Value
The hydroxyl value IOH is the number that expresses in milligrams the quantity of potassium hydroxide required to neutralise the acid combined by acylation in 1 g of the substance.
METHOD A
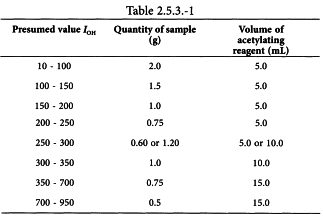
Introduce the quantity of the substance to be examined shown in Table 2.5.3.-1 (m g) into a 150 mL acetylation flask fitted with an air condenser, unless another quantity is prescribed in the monograph. Add the quantity of acetic anhydride solution R1 stated in Table 2.5.3.-1 and attach the air condenser.
Heat the flask in a water-bath for 1 h keeping the level of the water about 2.5 cm above the level of the liquid in the flask. Withdraw the flask and allow to cool. Add 5 mL of water R through the upper end of the condenser. If a cloudiness appears add sufficient pyridine R to clear it, noting the volume added. Shake the flask and replace in the water-bath for 10 min. Withdraw the flask and allow to cool. Rinse the condenser and the walls of the flask with 5 mL of alcohol R, previously neutralised to phenolphthalein solution R1. Titrate with 0.5 M alcoholic potassium hydroxide using 0.2 mL of phenolphthalein solution R1 as indicator (n1 mL of 0.5 M alcoholic potassium hydroxide). Carry out a blank test under the same conditions (n2 mL of 0.5 M alcoholic potassium hydroxide).
METHOD B
Introduce the prescribed quantity of the substance to be examined (m g) into a perfectly dry 5 mL conical flask fitted with a ground-glass or suitable plastic stopper and add 2.0 mL of propionic anhydride reagent R. Close the flask and shake gently to dissolve the substance. Allow to stand for 2 h unless otherwise prescribed. Remove the stopper and transfer the flask and its contents into a wide-mouthed 500 mL conical flask containing 25.0 mL of a 9 g/L solution of aniline R in cyclohexane R and 30 mL of glacial acetic acid R. Swirl the contents of the flask, allow to stand for 5 min, add 0.05 mL of crystal violet solution R and titrate with 0.1 M perchloric acid until an emerald-green colour is obtained (n1 mL of 0.1 M perchloric acid). Carry out a blank test under the same conditions (n2 mL of 0.1 M perchloric acid).
To take account of any water present, determine this (y per cent) by the semi-micro determination of water (2.5.12).
The hydroxyl value is then given by the equation: