Appendix XVI G. Microbiological Quality of Herbal Medicinal Products for Oral Use and Extracts Used in their Preparation
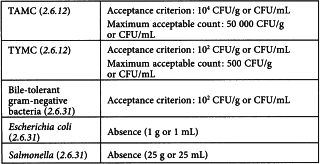
Microbial examination of non-sterile products is performed according to the methods given in general chapters 2.6.12, 2.6.13 and 2.6.31. Acceptance criteria based upon the total aerobic microbial count (TAMC) and the total combined yeasts/moulds count (TYMC) are given below.
Acceptance criteria are based on individual results or on the average of replicate counts when replicate counts are performed (e.g. direct plating methods).
A list of specified micro-organisms for which acceptance criteria are set can be found below. The list is not necessarily exhaustive and for a given preparation it may be necessary to test for other micro-organisms depending on the nature of the starting materials, the manufacturing process and the intended use.
Herbal medicinal products
Extracts
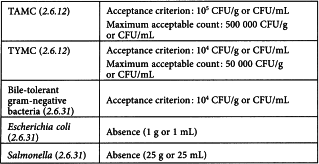
Extracts should fulfill the acceptance criteria for category B herbal medicinal products. However, where it can be demonstrated that the method of processing would not reduce the level of micro-organisms sufficiently to reach the category B criteria, the extracts shall meet the requirements for category C herbal medicinal products.
The recommended acceptance criteria apply to extracts that are to be incorporated into herbal medicinal products for oral use. More-stringent acceptance criteria may be required for extracts that are to be incorporated into pharmaceutical preparations to be administered by other routes in order to satisfy the acceptance criteria for the intended route of administration (5.1.4).
It is recognised that for some herbal medicinal products and extracts used in their preparation the criteria given above for TAMC, TYMC and bile-tolerant gram-negative bacteria cannot be met because of the typical level of microbial contamination. Less-stringent acceptance criteria may be applied on the basis of a risk assessment that takes account of qualitative and quantitative characterisation of the microbial contamination and the intended use of the herbal medicinal product or extract.
If it has been shown that none of the prescribed tests for a herbal medicinal product or extract will allow valid enumeration of micro-organisms at the level prescribed, a validated method with a limit of detection as close as possible to the indicated acceptance criterion is used.