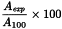Appendix XIX D. Containers for Blood and Blood Components
1. Sterile Plastic Containers for Human Blood and Blood Components
Plastic containers for the collection, storage, processing and administration of blood and its components are manufactured from one or more polymers, if necessary with additives. The composition and the conditions of manufacture of the containers are registered by the appropriate competent authorities in accordance with the relevant national legislation and international agreements.
When the composition of the materials of the different parts of the containers correspond to the appropriate specifications, their quality is controlled by the methods indicated in those specifications (see 3.1. Materials used for the manufacture of containers and subsections).
Materials other than those described in the Pharmacopoeia may be used provided that their composition is authorised by the competent authority and that the containers manufactured from them comply with the requirements prescribed for Sterile Plastic Containers for Human Blood and Blood Components.
In normal conditions of use the materials do not release monomers, or other substances, in amounts likely to be harmful nor do they lead to any abnormal modifications of the blood.
The containers may contain anticoagulant solutions, depending on their intended use, and are supplied sterile.
Each container is fitted with attachments suitable for the intended use. The container may be in the form of a single unit or the collecting container may be connected by one or more tubes to one or more secondary containers to allow separation of the blood components to be effected within a closed system.
The outlets are of a shape and size allowing for adequate connection of the container with the blood-giving equipment. The protective coverings on the blood-taking needle and on the appendages must be such as to ensure the maintenance of sterility. They must be easily removable but must be tamper-proof.
The capacity of the containers is related to the nominal capacity prescribed by the national authorities and to the appropriate volume of anticoagulant solution. The nominal capacity is the volume of blood to be collected in the container. The containers are of a shape such that when filled they may be centrifuged.
The containers are fitted with a suitable device for suspending or fixing which does not hinder the collection, storage, processing or administration of the blood.
The containers are enclosed in sealed, protective envelopes.
CHARACTERS
The container is sufficiently transparent to allow adequate visual examination of its contents before and after the taking of the blood and is sufficiently flexible to offer minimum resistance during filling and emptying under normal conditions of use. The container contains not more than 5 mL of air.
TESTS
Solution S1
Fill the container with 100 mL of a sterile, pyrogen-free 9 g/L solution of sodium chloride R. Close the container and heat it in an autoclave so that the contents are maintained at 110 °C for 30 min.
If the container to be examined contains an anticoagulant solution, first empty it, rinse the container with 250 mL of water for injections R at 20 ± 1 °C and discard the rinsings.
Solution S2
Introduce into the container a volume of water for injections R corresponding to the intended volume of anticoagulant solution. Close the container and heat it in an autoclave so that the contents are maintained at 110 °C for 30 min. After cooling, add sufficient water for injections R to fill the container to its nominal capacity.
If the container to be examined contains an anticoagulant solution, first empty it and rinse it as indicated above.
Resistance to centrifugation
Introduce into the container a volume of water R, acidified by the addition of 1 mL of dilute hydrochloric acid R, sufficient to fill it to its nominal capacity. Envelop the container with absorbent paper impregnated with a 1 in 5 dilution of bromophenol blue solution R1 or other suitable indicator and then dried. Centrifuge at 5000 g for 10 min. No leakage perceptible on the indicator paper and no permanent distortion occur.
Resistance to stretch
Introduce into the container a volume of water R, acidified by the addition of 1 mL of dilute hydrochloric acid R, sufficient to fill it to its nominal capacity. Suspend the container by the suspending device at the opposite end from the blood-taking tube and apply along the axis of this tube an immediate force of 20 N (2.05 kgf). Maintain the traction for 5 s. Repeat the test with the force applied to each of the parts for filling and emptying. No break and no deterioration occur.
Leakage
Place the container which has been submitted to the stretch test between two plates covered with absorbent paper impregnated with a 1 in 5 dilution of bromophenol blue solution R1 or other suitable indicator and then dried. Progressively apply force to the plates to press the container so that its internal pressure (i.e. the difference between the applied pressure and atmospheric pressure) reaches 67 kPa within 1 min. Maintain the pressure for 10 min. No signs of leakage are detectable on the indicator paper or at any point of attachment (seals, joints, etc.).
Vapour permeability
For a container containing an anticoagulant solution, fill with a volume of a 9 g/L solution of sodium chloride R equal to the volume of blood for which the container is intended.
For an empty container, fill with the same mixture of anticoagulant solution and sodium chloride solution. Close the container, weigh it and store it at 5 ± 1 °C in an atmosphere with a relative humidity of (50 ± 5) per cent for 21 days. At the end of this period the loss in mass is not greater than 1 per cent.
Emptying under pressure
Fill the container with a volume of water R at 5 ± 1 °C equal to the nominal capacity. Attach a transfusion set without an intravenous cannula to one of the connectors. Compress the container so as to maintain throughout the emptying an internal pressure (i.e the difference between the applied pressure and atmospheric pressure) of 40 kPa. The container empties in less than 2 min.
Speed of filling
Attach the container by means of the blood-taking tube fitted with the needle to a reservoir containing a suitable solution having a viscosity equal to that of blood, such as a 335 g/L solution of sucrose R at 37 °C. Maintain the internal pressure of the reservoir (i.e. the difference between the applied pressure and atmospheric pressure) at 9.3 kPa with the base of the reservoir and the upper part of the container at the same level. The volume of liquid which flows into the container in 8 min is not less than the nominal capacity of the container.
Resistance to temperature variations
Place the container in a suitable chamber having an initial temperature of 20-23 °C. Cool it rapidly in a deep-freeze to -80 °C and maintain it at this temperature for 24 h. Raise the temperature to 50 °C and maintain for 12 h. Allow to cool to room temperature. The container complies with the tests for resistance to centrifugation, resistance to stretch, leakage, vapour permeability emptying under pressure and speed of filling prescribed above.
Transparency
Fill the empty container with a volume equal to its nominal capacity of the primary opalescent suspension (2.2.1) diluted so as to have an absorbance (2.2.25) at 640 nm of 0.37 to 0.43 (dilution factor about 1 in 16). The cloudiness of the suspension must be perceptible when viewed through the bag, as compared with a similar container filled with water R.
Extractable matter
Tests are carried out by methods designed to simulate as far as possible the conditions of contact between the container and its contents which occur in conditions of use.
The conditions of contact and the tests to be carried out on the eluates are prescribed, according to the nature of the constituent materials, in the particular requirements for each type of container.
Haemolytic effects in buffered systems
Stock buffer solution Dissolve 90.0 g of sodium chloride R, 34.6 g of disodium hydrogen phosphate dodecahydrate R and 2.43 g of sodium dihydrogen phosphate R in water R and dilute to 1000 mL with the same solvent.
Buffer solution A0 To 30.0 mL of stock buffer solution add 10.0 mL of water R.
Buffer solution B0 To 30.0 mL of stock buffer solution add 20.0 mL of water R.
Buffer solution C0 To 15.0 mL of stock buffer solution add 85.0 mL of water R.
Introduce 1.4 mL of solution S2 into each of three centrifuge tubes. To tube I add 0.1 mL of buffer solution A0, to tube II add 0.1 mL of buffer solution B0 and to tube III add 0.1 mL of buffer solution C0. To each tube add 0.02 mL of fresh, heparinised human blood, mix well and warm on a water-bath at 30 ± 1 °C for 40 min. Use blood collected less than 3 h previously or blood collected into an anticoagulant citrate-phosphate-dextrose solution (CPD) less than 24 h previously.
Prepare three solutions containing, respectively:
3.0 mL of buffer solution A0 and 12.0 mL of water R (solution A1),
4.0 mL of buffer solution B0 and 11.0 mL of water R (solution B1),
4.75 mL of buffer solution B0 and 10.25 mL of water R (solution C1).
To tubes I, II and III add, respectively, 1.5 mL of solution A1, 1.5 mL of solution B1 and 1.5 mL of solution C1. At the same time and in the same manner, prepare three other tubes, replacing solution S2 by water R. Centrifuge simultaneously the tubes to be examined and the control tubes at exactly 2500 g in the same horizontal centrifuge for 5 min. After centrifuging, measure the absorbances (2.2.25) of the liquids at 540 nm using the stock buffer solution as compensation liquid. Calculate the haemolytic value as a percentage from the expression:
A100 | = | absorbance of tube III, |
Aexp | = | absorbance of tube I or II or of the corresponding control tubes. |
The solution in tube I gives a haemolytic value not greater than 10 per cent and the haemolytic value of the solution in tube II does not differ by more than 10 per cent from that of the corresponding control tube.
Sterility (2.6.1)
The containers comply with the test for sterility. Introduce aseptically into the container 100 mL of a sterile 9 g/L solution of sodium chloride and shake the container to ensure that the internal surfaces have been entirely wetted. Filter the contents of the container through a membrane filter and place the membrane in the appropriate culture medium, as prescribed in the test for sterility.
Pyrogens (2.6.8)
Solution S1 complies with the test for pyrogens. Inject 10 mL of the solution per kilogram of the rabbit’s mass.
Abnormal toxicity (2.6.9)
Solution S1 complies with the test for abnormal toxicity. Inject 0.5 mL of the solution into each mouse.
PACKAGING
The containers are packed in protective envelopes.
On removal from its protective envelope the container shows no leakage and no growth of micro-organisms. The protective envelope is sufficiently robust to withstand normal handling.
The protective envelope is sealed in such a manner that it cannot be opened and re-closed without leaving visible traces that the seal has been broken.
LABELLING
The labelling complies with the relevant national legislation and international agreements. The label states:
A part of the label is reserved for:
The label of the protective envelope or the label on the container, visible through the envelope, states:
The ink or other substance used to print the labels or the writing must not diffuse into the plastic material of the container and must remain legible up to the time of use.
2. Empty Sterile Containers of Plasticised Poly(Vinyl Chloride) for Human Blood and Blood Components
Unless otherwise authorised as described under Sterile plastic containers for human blood and blood components (3.2.3), the nature and composition of the material from which the containers are made comply with the requirements for Materials based on plasticised poly(vinyl chloride) for containers for human blood and blood components and for containers for aqueous solutions for intravenous infusion (3.1.1).
TESTS
They comply with the tests prescribed for Sterile plastic containers for human blood and blood components (3.2.3) and with the following tests to detect extractable matter.
Reference solution
Heat water for injections R in a borosilicate-glass flask in an autoclave at 110 °C for 30 min.
Acidity or alkalinity
To a volume of solution S2 corresponding to 4 per cent of the nominal capacity of the container add 0.1 mL of phenolphthalein solution R. The solution remains colourless. Add 0.4 mL of 0.01 M sodium hydroxide. The solution is pink. Add 0.8 mL of 0.01 M hydrochloric acid and 0.1 mL of methyl red solution R. The solution is orange-red or red.
Absorbance (2.2.25)
Maximum 0.30, determined between wavelengths of 230 nm and 250 nm on solution S2; maximum 0.10, determined between wavelengths of 251 nm and 360 nm on solution S2. Use the reference solution as the compensation liquid.
Oxidisable substances
Immediately after preparation of solution S2 (see 3.2.3), transfer to a borosilicate-glass flask a quantity corresponding to 8 per cent of the nominal capacity of the container. At the same time, prepare a blank using an equal volume of the freshly prepared reference solution in another borosilicate-glass flask. To each solution add 20.0 mL of 0.002 M potassium permanganate and 1 mL of dilute sulfuric acid R. Allow to stand protected from light for 15 min. To each solution add 0.1 g of potassium iodide R. Allow to stand protected from light for 5 min and titrate immediately with 0.01 M sodium thiosulfate, using 0.25 mL of starch solution R as indicator. The difference between the 2 titrations is not more than 2.0 mL.
Extractable di(2-ethylhexyl) phthalate
Extraction solvent, ethanol (96 per cent) R diluted with water R to have a relative density (2.2.5) of 0.9389 to 0.9395, measured with a pycnometer.
Stock solution Dissolve 0.100 g of di(2-ethylhexyl) phthalate R in the extraction solvent and dilute to 100.0 mL with the same solvent.
Standard solutions Into 5 separate 100 mL volumetric flasks, introduce respectively 1.0 mL, 2.0 mL, 5.0 mL, 10.0 mL and 20.0 mL of stock solution and dilute to 100.0 mL with extraction solvent.
Measure the absorbances (2.2.25) of the standard solutions at the absorption maximum at 272 nm, using the extraction solvent as compensation liquid and plot a curve of absorbance against the concentration of di(2-ethylhexyl) phthalate.
Extraction procedure Using the donor tubing and the needle or adaptor, fill the empty container with a volume equal to half the nominal volume with the extraction solvent, previously heated to 37 °C in a well-stoppered flask. Expel the air completely from the container and seal the donor tube. Immerse the filled container in a horizontal position in a water-bath maintained at 37 ± 1 °C for 60 ± 1 min without shaking. Remove the container from the water-bath, invert it gently 10 times and transfer the contents to a glass flask. Immediately measure the absorbance at the maximum at 272 nm, using the extraction solvent as compensation liquid.
Determine the concentration of di(2-ethylhexyl) phthalate in milligrams per 100 mL of extract from the calibration curve. The concentration does not exceed:
Chlorides (2.4.4)
Maximum 0.4 ppm, determined with solution S2.
Prepare the standard using a mixture of 1.2 mL of chloride standard solution (5 ppm Cl) R and 13.8 mL of water R.
Ammonium (2.4.1)
Maximum 2 ppm.
Dilute 5 mL of solution S2 to 14 mL with water R.
Residue on evaporation
Evaporate to dryness 100 mL of solution S2 in a borosilicate-glass beaker of appropriate capacity, previously heated to 105 °C. Evaporate to dryness in the same conditions 100 mL of the reference solution (blank test). Dry to constant mass at 100-105 °C. The residue from solution S2 weighs a maximum of 3 mg, allowing for the blank test.
PACKAGING
See Sterile plastic containers for human blood and blood components (3.2.3).
LABELLING
See Sterile plastic containers for human blood and blood components (3.2.3).
3. Sterile Containers of Plasticised Poly(Vinyl Chloride) for Human Blood Containing Anticoagulant Solution
Sterile plastic containers containing an anticoagulant solution complying with the monograph Anticoagulant and preservative solutions for human blood (0209) are used for the collection, storage and administration of blood. Before filling they comply with the description and characters given under Empty sterile containers of plasticised poly(vinyl chloride) for human blood and blood components (3.2.4).
Unless otherwise authorised as described under Sterile plastic containers for human blood and blood components (3.2.3), the nature and composition of the material from which the containers are made should comply with the requirements prescribed for Materials based on plasticised poly(vinyl chloride) for containers for human blood and blood components and for containers for aqueous solutions for intravenous infusion (3.1.1).
TESTS
They comply with the tests prescribed for Sterile plastic containers for human blood and blood components (3.2.3) and with the following tests to measure the volume of anticoagulant solution and to detect extractable matter.
Volume of anticoagulant solution
Empty the container, collecting the anticoagulant solution in a graduated cylinder. The volume does not differ by more than ± 10 per cent from the stated volume.
Spectrophotometric examination (2.2.25)
Measure the absorbance of the anticoagulant solution from the container between 250 nm and 350 nm, using as the compensation liquid an anticoagulant solution of the same composition that has not been in contact with a plastic material. The absorbance at the maximum at 280 nm is not greater than 0.5.
Extractable di(2-ethylhexyl) phthalate
Carefully remove the anticoagulant solution by means of the flexible transfer tube. Using a funnel fitted to the tube, completely fill the container with water R, leave in contact for 1 min squeezing the container gently, then empty completely. Repeat the rinsing.
The container, so emptied and rinsed, complies with the test for extractable di(2-ethylhexyl) phthalate prescribed for Empty sterile plastic containers of plasticised poly(vinyl chloride) for human blood and blood components (3.2.4).
PACKAGING AND LABELLING
See Sterile plastic containers for human blood and blood components (3.2.3).