Dexamethasone Tablets
Action and use
Glucocorticoid.
Definition
Dexamethasone Tablets contain Dexamethasone.
Content of dexamethasone, C22H29FO5
95.0 to 105.0% of the stated amount.
Carry out all of the following procedures protected from light.
Identification
Mix a quantity of the powdered tablets containing 20 mg of Dexamethasone with 5 mL of 0.1m sodium hydroxide, add 50 mL of dichloromethane and mix with the aid of ultrasound for 20 minutes, filter and evaporate to dryness using a rotary evaporator. Dry the residue at 105° for 2 hours. The infrared absorption spectrum of the dried residue, Appendix II A, is concordant with the reference spectrum of dexamethasone (RS 089).
Tests
Related substances
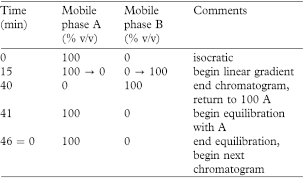
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
Mobile phase A15% v/v acetonitrile.
Mobile phase B acetonitrile.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between methylprednisolone and dexamethasone is at least 2.8.
In the chromatogram obtained with solution (1):
the area of any secondary peak is not greater than 0.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%);
the sum of the areas of all the secondary peaks is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1.0%).
Disregard any peak due to mobile phase A and any peak with an area less than the area of the principal peak in the chromatogram obtained with reference solution (4) (0.05%).
Uniformity of content
Tablets containing less than 2 mg and or less than 2% w/w of Dexamethasone comply with the requirements stated under Tablets using the following method of analysis. Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
47 volumes of methanol and 53 volumes of water.
Calculate the content of C22H29FO5 in each tablet using the declared content of C22H29FO5 in dexamethasone BPCRS.
Assay
For tablets containing less than 2 mg and/or less than 2% w/w of Dexamethasone
Use the average of the individual results determined in the test for Uniformity of content.
For tablets containing 2 mg or more and 2% w/w of Dexamethasone
Weigh and powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
The chromatographic conditions described under Uniformity of content may be used.
Calculate the content of C22H29FO5 in the tablets using the declared content of C22H29FO5 in dexamethasone BPCRS.
Storage
Dexamethasone Tablets should be protected from light.