Dipyridamole Tablets
Action and use
Adenosine reuptake inhibitor; inhibitor of platelet aggregation.
Definition
Dipyridamole Tablets contain Dipyridamole. They are coated.
Content of dipyridamole, C24H40N8O4
95.0 to 105.0% of the stated amount.
Identification
Shake a quantity of the powdered tablets containing 50 mg of Dipyridamole with 20 mL of dichloromethane, filter and evaporate to dryness. The infrared absorption spectrum of the residue, Appendix II A, is concordant with the reference spectrum of dipyridamole (RS 108).
TESTS
Related substances
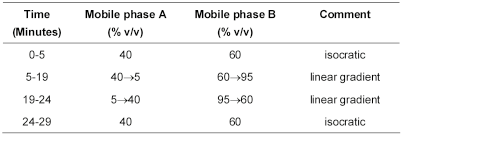
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in amber glassware and protected from light.
The test is not valid unless the chromatogram obtained with solution (3) closely resembles the chromatogram supplied with dipyridamole for peak identification EPCRS;
In the chromatogram obtained with solution (1):
identify any peak corresponding to impurity B and multiply the area of this peak by a correction factor of 1.7;
the area of any peak corresponding to impurity A, B, C, D or E is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.5%);
the area of any other secondary peak not greater than 0.4 times the area of the principal peak in the chromatogram obtained with solution (2) (0.2%);
the sum of the areas of any such peaks is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) (1%).
Disregard any peak with an area less than the principal peak in the chromatogram obtained with solution (4) (0.05%).
Assay
Weigh and powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions in amber glassware and protected from light.
The chromatographic conditions described under the Related substances test may be used.
The test is not valid unless the chromatogram obtained with solution (3) closely resembles the chromatogram supplied with dipyridamole for peak identification EPCRS;
Calculate the content of C24H40N8O4 in the tablets using the declared content of C24H40N8O4 in dipyridamole BPCRS.
Impurities
The impurities limited by the requirements of this monograph include those listed under Dipyridamole.