Hydroxychloroquine Tablets
Action and use
Used in the treatment of rheumatoid arthritis.
Definition
Hydroxychloroquine Tablets contain Hydroxychloroquine Sulfate. They are coated.
Content of hydroxychloroquine sulfate, C18H26ClN3O,H2SO4
92.5 to 107.5% of the stated amount.
Identification
TEST
Disintegration
Maximum time, 45 minutes, Appendix XII A1.
Related substances
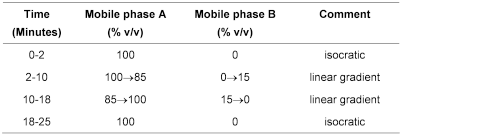
Carry out the method for liquid chromatography, Appendix III D, using the following solutions of the substance being examined in mobile phase A.
Mobile phase A0.2 volumes of orthophosphoric acid, 10 volumes of acetonitrile and 90 volumes of water.
Mobile phase B0.1 volumes of orthophosphoric acid, 20 volumes of water and 80 volumes of acetonitrile.
The test is not valid unless, in the chromatogram obtained with solution (5), the resolution between 2-[4-[(7-chloro-4-quinolinyl)amino]pentyl] amino ethanol and hydroxychloroquine is at least 1.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to 2-[4-[(7-chloro-4-quinolinyl)amino]pentyl]aminoethanol is not greater than the area of the principal peak in the chromatogram obtained with solution (4) (0.5%);
the area of any other secondary peak is not greater than the principal peak in the chromatogram obtained with solution (2) (0.5%).
the sum of the areas of any other secondary peaks is not greater than twice the principal peak in the chromatogram obtained with solution (2) (1.0%).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (3) (0.05%).
Assay
Carry out the method for liquid chromatography, Appendix III D, using the following solutions of the substance being examined in mobile phase A.
The chromatographic procedure may be carried out using the conditions described under the Related substances test.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between 2-[4-[(7-chloro-4-quinolinyl)amino]pentyl] amino ethanol and hydroxychloroquine is at least 1.0.
Calculate the total content of hydroxychloroquine sulfate, C18H26ClN3O,H2SO4, in the tablets using the declared content of C18H26ClN3O,H2SO4 in hydroxychloroquine sulfate BPCRS.