Oxybutynin Tablets
Action and use
Anticholinergic.
Definition
Oxybutynin Tablets contain Oxybutynin Hydrochloride.
Content of oxybutynin hydrochloride, C22H31NO3,HCl
95.0 to 105.0% of the stated amount.
Identification
To a quantity of the powdered tablets containing 25 mg of Oxybutynin Hydrochloride add sufficient 2m sodium hydroxide to adjust to pH 12.0 and extract with four 20-mL quantities of hexane. Filter the collected hexane layers through anhydrous sodium sulfate (Whatman GF/C is suitable). Evaporate the filtrate to dryness under a current of nitrogen, to yield a clear, sticky liquid residue. The infrared absorption spectrum of the residue, Appendix II A, is concordant with the reference spectrum of oxybutynin (RS442).
Tests
Dissolution
Comply with the requirements for Monographs of the British Pharmacopoeia in the dissolution test for tablets and capsules, Appendix XII B1.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
The chromatographic conditions described under Assay may be used.
Calculate the total content of Oxybutynin Hydrochloride, C22H31NO3,HCl, in the medium from the chromatograms obtained and using the declared content of C22H31NO3,HCl in oxybutynin hydrochloride BPCRS.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
The test is not valid unless, in the chromatogram obtained with solution (5), the resolution factor between the peaks due to oxybutynin hydrochloride and oxybutynin impurity A is at least 10.0.
In the chromatogram obtained with solution (1);
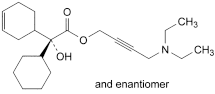
the area of any peak corresponding to oxybutynin impurity A is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1.5%);
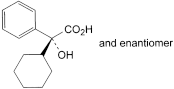
the area of any peak corresponding to phenylcyclohexylglycolic acid is not greater than half the area of the principal peak in the chromatogram obtained with solution (3) (0.5%);
the area of any other secondary peak is not greater than 0.4 times the area of the principal peak in the chromatogram obtained with solution (4) (0.2%);
the sum of the areas of any such secondary peaks is not greater than the area of the principal peak in the chromatogram obtained with solution (4) (0.5%).
Disregard any peak with an area less than 0.1 times the area of the principal peak in the chromatogram obtained with solution (4) (0.05%).
Assay
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
300 volumes of acetonitrile R1 and 700 volumes of a 0.48% w/v solution of anhydrous potassium dihydrogen orthophosphate previously adjusted to pH 3.0 to 3.5 with orthophosphoric acid.
Calculate the content of C22H31NO3,HCl in the tablets using the declared content of C22H31NO3,HCl in oxybutynin hydrochloride BPCRS.
Impurities
The impurities limited by the requirements of this monograph include: