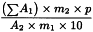Boldo Leaf Dry Extract
Ph Eur
DEFINITION
Extract produced from Boldo leaf (1396).
Content
PRODUCTION
The extract is produced from the herbal drug by a suitable procedure using either hot water at not less than 65 °C or a hydroalcoholic solvent equivalent in strength to ethanol (45-75 per cent V/V).
CHARACTERS
Appearance
Brown or greenish-brown, hygroscopic powder.
IDENTIFICATION
Thin-layer chromatography (2.2.27).
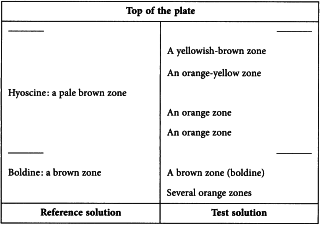
Test solution To 0.5 g of the extract to be examined add 1 mL of hydrochloric acid R and 20 mL of water R. Sonicate for 10 min. Transfer the liquid to a separating funnel and make alkaline with 2 mL of dilute ammonia R1. Shake with 2 quantities, each of 20 mL, of methylene chloride R. Evaporate the combined organic layers to dryness. Dissolve the residue in 1 mL of methanol R.
Reference solution Dissolve 2 mg of boldine R and 10 mg of hyoscine hydrobromide R in 5 mL of methanol R.
PlateTLC silica gel plate R (5-40 µm) [or TLC silica gel plate R (2-10 µm)].
Mobile phasediethylamine R, methanol R, toluene R (10:10:80 V/V/V).
Application 20 µL [or 3 µL] as bands of 15 mm [or 8 mm].
Development Over a path of 15 cm [or 6 cm].
Drying In air.
Detection Treat with potassium iodobismuthate solution R2, allow to dry in air for 5 min and treat with sodium nitrite solution R; examine in daylight after 30 min.
Results See below the sequence of zones present in the chromatograms obtained with the reference solution and the test solution. Furthermore, other faint zones may be present in the chromatogram obtained with the test solution.
TESTS
Water (2.5.12)
Maximum 5.0 per cent, determined on 0.5 g.
ASSAY
Liquid chromatography (2.2.29).
Test solution Disperse 1.000 g of the extract to be examined in 50 mL of dilute hydrochloric acid R and sonicate for 10 min. Transfer to a separating funnel and wash with 10 mL of a mixture of equal volumes of ethyl acetate R and hexane R. Discard the organic layer. Adjust the aqueous phase to pH 9.5 with concentrated ammonia R. After cooling, shake successively with 100 mL, 50 mL, and a further 50 mL of methylene chloride R, taking care not to form an emulsion. If necessary, centrifuge at 1200 g for 10 min. Combine the lower layers and evaporate to dryness under reduced pressure. Dissolve the residue in the mobile phase and dilute to 10.0 mL with the mobile phase.
Reference solution (a) Dissolve 12.0 mg of boldine CRS in the mobile phase and dilute to 100.0 mL with the mobile phase.
Reference solution (b) Disperse 0.5 g of boldo leaf dry extract HRS in 50 mL of dilute hydrochloric acid R and sonicate for 10 min. Transfer to a separating funnel and wash with 10 mL of a mixture of equal volumes of ethyl acetate R and hexane R. Discard the organic layer. Adjust the aqueous phase to pH 9.5 with concentrated ammonia R. After cooling, shake successively with 100 mL, 50 mL, and a further 50 mL of methylene chloride R, taking care not to form an emulsion. If necessary, centrifuge at 1200 g for 10 min. Combine the lower layers and evaporate to dryness under reduced pressure. Dissolve the residue in the mobile phase and dilute to 10.0 mL with the mobile phase.
Solution A Mix 0.2 mL of diethylamine R and 99.8 mL of acetonitrile R.
Solution B Mix 0.2 mL of diethylamine R and 99.8 mL of water R and adjust to pH 3 with anhydrous formic acid R.
Mobile phase Solution A, solution B (16:84 V/V).
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 304 nm.
Injection 20 µL.
Identification of peaks Use the chromatogram supplied with boldo leaf dry extract HRS and the chromatogram obtained with reference solution (b) to identify the peaks due to alkaloids 1, 3, 4, 5 and 6; use the chromatogram obtained with reference solution (a) to identify the peak due to boldine.
Relative retention With reference to boldine (retention time = about 6 min): alkaloid 1 = about 0.9; alkaloid 3 = about 1.8; alkaloid 4 = about 2.0; alkaloid5 = about 2.9; alkaloid6 = about 3.1. Additional peaks may be present.
Calculate the percentage content of total alkaloids, expressed as boldine, using the following expression:
ΣA1 | = | sum of the areas of the peaks due to alkaloids 1, 3, 4, 5 and 6 and the peak due to boldine in the chromatogram obtained with the test solution; |
A2 | = | area of the peak due to boldine in the chromatogram obtained with reference solution (a); |
m1 | = | mass of the extract to be examined used to prepare the test solution, in grams; |
m2 | = | mass of boldine CRS used to prepare reference solution (a), in grams; |
p | = | percentage content of boldine in boldine CRS. |
Ph Eur