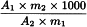Devil’s Claw Dry Extract
Ph Eur
DEFINITION
Dry extract obtained from Devil′s claw root (1095).
Content
Minimum 1.5 per cent of harpagoside (C24H30O11; Mr 494.5) (dried extract).
PRODUCTION
The extract is produced from the herbal drug by an appropriate procedure using either water or a hydroalcoholic solvent that is at most equivalent in strength to ethanol (95 per cent V/V).
CHARACTERS
Appearance
Light brown powder.
IDENTIFICATION
Thin-layer chromatography (2.2.27).
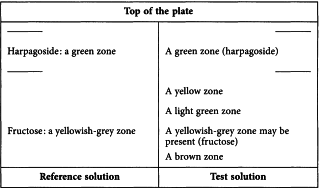
Test solution To 1.0 g of the extract to be examined add 10 mL of methanol R and heat in a water-bath at 60 °C for 10 min. Cool and filter.
Reference solution Dissolve 1.0 mg of harpagoside R and 2.5 mg of fructose R in 1.0 mL of methanol R.
PlateTLC silica gel plate R.
Mobile phasewater R, methanol R, ethyl acetate R (8:15:77 V/V/V).
Application 20 µL as bands.
Development Over a path of 10 cm.
Drying In a current of warm air.
Detection Spray with a 10 g/L solution of phloroglucinol R in ethanol (96 per cent) R and then with hydrochloric acid R; heat at 80 °C for 5-10 min and examine in daylight.
Results See below the sequence of zones present in the chromatograms obtained with the reference solution and the test solution. Furthermore, other faint zones may be present in the chromatogram obtained with the test solution.
ASSAY
Liquid chromatography (2.2.29).
Test solution Introduce 0.350 g of the extract to be examined into a 100 mL volumetric flask, add 90 mL of methanol R and sonicate for 20 min. Cool to room temperature, dilute to 100.0 mL with methanol R and filter through a membrane filter (nominal pore size 0.2 µm).
Reference solution Dissolve the contents of 1 vial of harpagoside CRS in methanol R and dilute to 10.0 mL with the same solvent.
Mobile phasemethanol R, water R (50:50 V/V).
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 278 nm.
Injection 10 µL.
Run time 3 times the retention time of harpagoside.
Retention time Harpagoside = about 7 min.
Calculate the percentage content of harpagoside using the following expression:
A1 | = | area of the peak due to harpagoside in the chromatogram obtained with the test solution; |
A2 | = | area of the peak due to harpagoside in the chromatogram obtained with the reference solution; |
m1 | = | mass of the extract to be examined used to prepare the test solution, in grams; |
m2 | = | mass of harpagoside contained in 1 vial of harpagoside CRS, in grams. |
Ph Eur