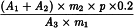Valerian Dry Aqueous Extract
Ph Eur
DEFINITION
Extract produced from Valerian root (0453).
Content
Minimum 0.02 per cent of sesquiterpenic acids, expressed as valerenic acid (C15H22O2; Mr 234.3) (dried extract).
PRODUCTION
The extract is produced from the herbal drug by a suitable procedure using water at not less than 60 °C.
CHARACTERS
Appearance
Brown or brownish, hygroscopic powder.
IDENTIFICATION
Thin-layer chromatography (2.2.27).
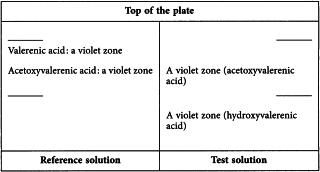
Test solution Suspend 1.0 g of the extract to be examined in 10 mL of methanol R and sonicate for 10 min. Filter the supernatant through a membrane filter (nominal pore size 0.45 µm). Use the filtrate as the test solution.
Reference solution Dissolve 5 mg of acetoxyvalerenic acid R and 5 mg of valerenic acid R in 20 mL of methanol R.
PlateTLC silica gel plate R (5-40 µm) [or TLC silica gel plate R (2-10 µm)].
Mobile phaseglacial acetic acid R, ethyl acetate R, cyclohexane R (2:38:60 V/V/V).
Application 20 µL [or 5 µL] as bands of 10 mm [or 8 mm].
Development Over a path of 10 cm [or 6 cm].
Drying In air.
Detection Spray with anisaldehyde solution R and heat at 100-105 °C for 5-10 min; examine in daylight.
Results See below the sequence of zones present in the chromatograms obtained with the reference solution and the test solution. A faint violet zone due to valerenic acid may be present in the chromatogram obtained with the test solution. Furthermore, other zones may be present in the chromatogram obtained with the test solution.
TESTS
Loss on drying (2.8.17)
Maximum 6.0 per cent.
ASSAY
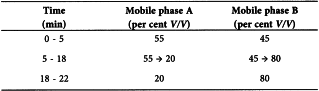
Liquid chromatography (2.2.29).
Solvent mixturemethanol R, water R (50:50 V/V).
Test solution In a 300 mL conical flask suspend 1.00 g of the extract to be examined in 40 mL of water R whilst swirling. Add 40 mL of methanol R and swirl for 1 h at 200 r/min. Filter the suspension into a volumetric flask and rinse the conical flask with 3 quantities, each of 5 mL, of the solvent mixture. Dilute to 100.0 mL with the solvent mixture.
Reference solution (a) Dissolve a quantity of valerian dry extract HRS corresponding to 1.0 mg of valerenic acid in methanol R and dilute to 10.0 mL with the same solvent. Sonicate for 10 min and filter through a membrane filter (nominal pore size 0.45 µm).
Reference solution (b) Dilute 1.0 mL of reference solution (a) to 50.0 mL with methanol R.
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 220 nm.
Injection 20 µL.
Identification of peaks Use the chromatogram supplied with valerian dry extract HRS and the chromatogram obtained with reference solution (a) to identify the peaks due to acetoxyvalerenic acid and hydroxyvalerenic acid.
Relative retention With reference to valerenic acid (retention time = about 19 min): hydroxyvalerenic acid = about 0.2; acetoxyvalerenic acid = about 0.5.
Calculate the percentage content of sesquiterpenic acids, expressed as valerenic acid, using the following expression:
A1 | = | area of the peak due to hydroxyvalerenic acid in the chromatogram obtained with the test solution; |
A2 | = | area of the peak due to acetoxyvalerenic acid in the chromatogram obtained with the test solution; |
A3 | = | area of the peak due to valerenic acid in the chromatogram obtained with reference solution (b); |
m1 | = | mass of the extract to be examined used to prepare the test solution, in grams; |
m2 | = | mass of valerian dry extract HRS used to prepare reference solution (a), in grams; |
p | = | percentage content of valerenic acid in valerian dry extract HRS. |
Ph Eur