Appendix XX H. Polyethylene Terephthalate for Containers for Preparations not for Parenteral Use
DEFINITION
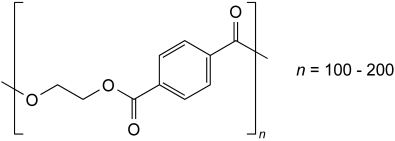
Polyethylene terephthalate is obtained from the polymerisation of terephthalic acid or dimethyl terephthalate with ethylene glycol. Isophthalic acid, dimethyl isophthalate, 1,4-bis(hydroxymethyl)cyclohexane (cyclohexane-1,4-dimethanol) or diethylene glycol may be used in the polymerisation. It may contain not more than 0.5 per cent of silica or silicates and colouring matter approved by the competent authority.
PRODUCTION
The manufacturing process is validated to demonstrate that the residual acetaldehyde content is not greater than 10 ppm in the granules.
CHARACTERS
Appearance Clear or opaque granules.
Solubility Practically insoluble in water, in ethanol (96 per cent) and in methylene chloride. It is hydrolysed by strong bases.
IDENTIFICATION
TESTS
If necessary, cut out samples for testing to a maximum size of 1 cm per side.
Solution S1
Place 10.0 g of the material to be examined in a borosilicate glass flask with a ground-glass neck. Add 200 mL of water R and heat at 50 °C for 5 h. Allow to cool and decant the solution. Use solution S1 within 4 h of its preparation.
Solution S2
Place 10 g of the material to be examined in a borosilicate glass flask with a ground-glass neck. Add 100 mL of ethanol (96 per cent) R and heat at 50 °C for 5 h. Allow to cool and decant the solution. Use solution S2 within 4 h of its preparation.
Solution S3
Place 20 g of the material to be examined in a borosilicate glass flask with a ground-glass neck. Add 50 mL of 0.1 M hydrochloric acid and heat at 50 °C for 5 h. Allow to cool and decant the solution. Use solution S3 within 4 h of its preparation.
Solution S4
Place 20 g of the material to be examined into a borosilicate glass flask with a ground-glass neck. Add 50 mL of 0.01 M sodium hydroxide and heat at 50 °C for 5 h. Allow to cool and decant. Use solution S4 within 4 h of its preparation.
Appearance of solution S1
Solution S1 is clear (2.2.1).
Appearance of solution S2
Solution S2 is clear (2.2.1) and colourless (2.2.2, Method II).
Acidity or alkalinity
To 50 mL of solution S1 add 0.15 mL of BRP indicator solution R. The solution turns yellow. Not more than 0.5 mL of 0.01 M sodium hydroxide is required to change the colour of the indicator to blue. To another 50 mL of solution S1 add 0.2 mL of methyl orange solution R. The solution turns yellow. Not more than 0.5 mL of 0.01 M hydrochloric acid is required to reach the beginning of the colour change of the indicator to orange.
Absorbance of solution S1 (2.2.25)
Maximum 0.20 between 220 nm and 340 nm. In addition, for coloured polyethylene terephthalate: maximum 0.05 between 400 nm to 800 nm.
Absorbance of solution S2 (2.2.25)
Maximum 0.05 between 400 nm and 800 nm.
Reducing substances
Add 2 mL of 0.5 M sulfuric acid and 20.0 mL of 0.002 M potassium permanganate to 20.0 mL of solution S1. Boil for 3 min. Cool immediately to ambient temperature. Add 1 g of potassium iodide R, 0.25 mL of starch solution R as indicator and titrate with 0.01 M sodium thiosulfate. Perform a blank titration using 20.0 mL of water R. The difference in volume used in the 2 titrations is not greater than 0.5 mL.
Substances soluble in dioxan
Maximum 3 per cent.
Place 2 g of the material to be examined in a borosilicate glass flask with a ground-glass neck. Add 20 mL of dioxan R and heat under reflux for 2 h. Evaporate 10 mL of the solution to dryness on a water-bath and then dry the residue at 100-105 °C. The residue weighs a maximum of 30 mg.
Extractable aluminium
Maximum 1 ppm.
Inductively coupled plasma-atomic emission spectrometry (2.2.57).
Test solution Solution S3.
Reference solutions Prepare the reference solutions using aluminium standard solution (200 ppm Al) R, diluting with 0.1 M hydrochloric acid.
Wavelength 396.15 nm, the spectral background being taken at 396.25 nm.
Verify the absence of aluminium in the 0.1 M hydrochloric acid used.
Extractable antimony
Maximum 1 ppm.
Inductively coupled plasma-atomic emission spectrometry (2.2.57).
Test solution Solution S4.
Reference solutions Prepare the reference solutions using antimony standard solution (100 ppm Sb) R, diluting with 0.01 M sodium hydroxide.
Wavelength 231.15 nm or 217.58 nm, the spectral background being taken at 231.05 nm.
Extractable barium
Maximum 1 ppm.
Inductively coupled plasma-atomic emission spectrometry (2.2.57).
Test solution Solution S3.
Reference solutions Prepare the reference solutions using barium standard solution (50 ppm Ba) R, diluting with 0.1 M hydrochloric acid.
Wavelength 455.40 nm, the spectral background being taken at 455.30 nm.
Verify the absence of barium in the 0.1 M hydrochloric acid used.
Extractable cobalt
Maximum 1 ppm.
Inductively coupled plasma-atomic emission spectrometry (2.2.57).
Test solution Solution S3.
Reference solutions Prepare the reference solutions using cobalt standard solution (100 ppm Co) R, diluting with 0.1 M hydrochloric acid.
Wavelength 228.62 nm, the spectral background being taken at 228.50 nm.
Verify the absence of cobalt in the 0.1 M hydrochloric acid used.
Extractable germanium
Maximum 1 ppm.
Inductively coupled plasma-atomic emission spectrometry (2.2.57).
Test solution Solution S4.
Reference solutions Prepare the reference solutions using germanium standard solution (100 ppm Ge) R, diluting with 0.01 M sodium hydroxide.
Wavelength 206.87 nm or 265.12 nm, the spectral background being taken at 206.75 nm.
Extractable manganese
Maximum 1 ppm.
Inductively coupled plasma-atomic emission spectrometry (2.2.57).
Test solution Solution S3.
Reference solutions Prepare the reference solutions using manganese standard solution (100 ppm Mn) R, diluting with 0.1 M hydrochloric acid.
Wavelength 257.61 nm, the spectral background being taken at 257.50 nm.
Verify the absence of manganese in the 0.1 M hydrochloric acid used.
Extractable titanium
Maximum 1 ppm.
Inductively coupled plasma-atomic emission spectrometry (2.2.57).
Test solution Solution S3.
Reference solutions Prepare the reference solutions using titanium standard solution (100 ppm Ti) R, diluting with 0.1 M hydrochloric acid.
Wavelength 323.45 nm or 334.94 nm, the spectral background being taken at 323.35 nm.
Verify the absence of titanium in the 0.1M hydrochloric acid used.
Extractable zinc
Maximum 1 ppm.
Inductively coupled plasma-atomic emission spectrometry (2.2.57).
Test solution Solution S3.
Reference solutions Prepare the reference solutions using zinc standard solution (100 ppm Zn) R, diluting with 0.1 M hydrochloric acid.
Wavelength 213.86 nm, the spectral background being taken at 213.75 nm.
Verify the absence of zinc in the 0.1 M hydrochloric acid used.
Sulfated ash (2.4.14)
Maximum 0.5 per cent determined on 1.0 g.