SC IV J. Control of Impurities in Substances for Pharmaceutical Use
Preamble
The monographs of the European Pharmacopoeia on substances for pharmaceutical use are designed to ensure acceptable quality for users. The role of the Pharmacopoeia in public health protection requires that adequate control of impurities be provided by monographs. The quality required is based on scientific, technical and regulatory considerations.
Requirements concerning impurities are given in specific monographs and in the general monograph Substances for pharmaceutical use (2034). Specific monographs and the general monograph are complementary: specific monographs prescribe acceptance criteria for impurities whereas the general monograph deals with the need for qualification, identification and reporting of any organic impurities that occur in active substances.
The thresholds for reporting, identification and qualification contained in the general monograph Substances for pharmaceutical use (2034) apply to all related substances. However, if a monograph does not contain a related substances test based on a quantitative method, any new impurities occurring above a threshold may be overlooked since the test is not capable to detect those impurities.
The provisions of the Related substances section of the general monograph Substances for pharmaceutical use (2034), notably those concerning thresholds, do not apply to excipients; also excluded from the provisions of this section are: biological and biotechnological products; oligonucleotides; radiopharmaceuticals; fermentation products and semi-synthetic products derived therefrom; herbal products and crude products of animal and plant origin. Although the thresholds stated in the general monograph do not apply, the general concepts of reporting, identification (wherever possible) and qualification of impurities are equally valid for these classes.
Basis for the elaboration of monographs of the European Pharmacopoeia
European Pharmacopoeia monographs are elaborated on substances that are present in medicinal products that have been authorised by the competent authorities of Parties to the European Pharmacopoeia Convention. Consequently, these monographs do not necessarily cover all sources of substances for pharmaceutical use on the world market.
Organic and inorganic impurities present in those substances that have been evaluated by the competent authorities are qualified with respect to safety at the maximum authorised content (at the maximum daily dose) unless new safety data that become available following evaluation justify lower limits.
European Pharmacopoeia monographs on substances for pharmaceutical use are elaborated by groups of experts and working parties collaborating with national pharmacopoeia authorities, the competent authorities for marketing authorisation, national control laboratories and the European Pharmacopoeia laboratory; they are also assisted by the producers of the substances and/or the pharmaceutical manufacturers that use these substances.
Control of impurities in substances for pharmaceutical use
The quality with respect to impurities is controlled by a set of tests within a monograph. These tests are intended to cover organic and inorganic impurities that are relevant in view of the sources of active substances in authorised medicinal products.
Control of residual solvents is provided by the general monograph Substances for pharmaceutical use (2034) and general chapter 5.4. Residual solvents. The certificate of suitability of a monograph of the European Pharmacopoeia for a given source of a substance indicates the residual solvents that are controlled together with the specified acceptance criteria and the validated control method where this differs from those described in general chapter 2.4.24. Identification and control of residual solvents.
Monographs on organic chemicals usually have a test entitled “Related substances” that covers relevant organic impurities. This test may be supplemented by specific tests where the general test does not control a given impurity or where there are particular reasons (for example, safety reasons) for requiring special control.
Where a monograph has no Related substances (or equivalent) test but only specific tests, the user of a substance must nevertheless ensure that there is suitable control of organic impurities; those occurring above the identification threshold are to be identified (wherever possible) and, unless justified, those occurring above the qualification threshold are to be qualified (see also under Recommendations to users of monographs of active substances).
Where the monograph covers substances with different impurity profiles, it may have a single related substances test to cover all impurities mentioned in the Impurities section or several tests may be necessary to give control of all known profiles. Compliance may be established by carrying out only the tests relevant to the known impurity profile for the source of the substance.
Instructions for control of impurities may be included in the Production section of a monograph, for example where the only analytical method appropriate for the control of a given impurity is to be performed by the manufacturer since the method is too technically complex for general use or cannot be applied to the final drug substance and/or where validation of the production process (including the purification step) will give sufficient control.
Impurities section in monographs on active substances
The Impurities section in a monograph includes impurities (chemical structure and name wherever possible), which are usually organic, that are known to be detected by the tests prescribed in the monograph. It is based on information available at the time of elaboration or revision of the monograph and is not necessarily exhaustive. The section includes specified impurities and, where so indicated, other detectable impurities.
Specified impurities Have an acceptance criterion not greater than that authorised by the competent authorities.
Other detectable impurities Are potential impurities with a defined structure but not known to be normally present above the identification threshold in substances used in medicinal products that have been authorised by the competent authorities of Parties to the Convention. They are given in the Impurities section for information.
Where an impurity other than a specified impurity is found in an active substance it is the responsibility of the user of the substance to check whether it has to be identified/qualified, depending on its content, nature, maximum daily dose and relevant identification/qualification threshold, in accordance with the general monograph on Substances for pharmaceutical use (2034), Related substances section.
It should be noted that specific thresholds are applied to substances exclusively for veterinary use.
Interpretation of the test for related substances in the monographs on active substances
A specific monograph on a substance for pharmaceutical use is to be read and interpreted in conjunction with the general monograph on Substances for pharmaceutical use (2034).
Where a general acceptance criterion for impurities (“any other impurity”, “other impurities”, “any impurity”) equivalent to a nominal content greater than the applicable identification threshold (see the general monograph on Substances for pharmaceutical use (2034)) is prescribed, this is valid only for specified impurities mentioned in the Impurities section. The need for identification (wherever possible), reporting, specification and qualification of other impurities that occur must be considered according to the requirements of the general monograph. It is the responsibility of the user of the substance to determine the validity of the acceptance criteria for impurities not mentioned in the Impurities section and for those indicated as other detectable impurities.
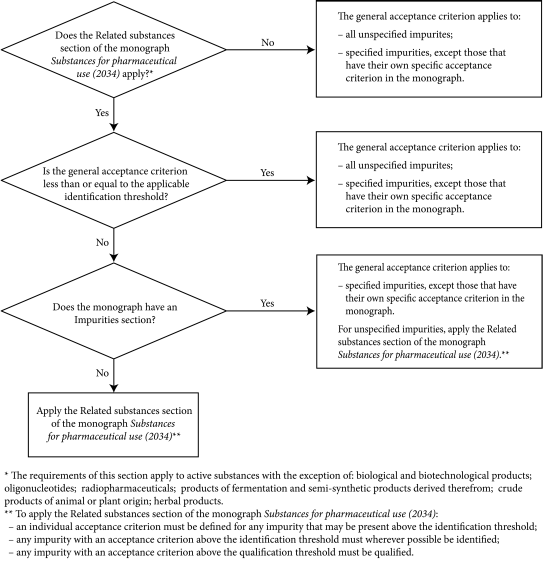
Acceptance criteria for the related substances test are presented in different ways in existing monographs; the decision tree (Figure 5.10.-1) may be used as an aid in the interpretation of general acceptance criteria and their relation with the Impurities section of the monograph.
General acceptance criteria for “other” impurities are expressed in various ways in the monographs: “any other impurity”, “other impurities”, “any impurity”, “any spot”, “any band”, etc. The general acceptance criteria may apply to certain specified impurities only or to unspecified impurities and certain specified impurities, depending on the nature of the active substance and the applicable identification threshold. Pending editorial adaptation of already published monographs using unequivocal terminology, the decision tree (Figure 5.10.-1) may be used to determine the acceptance criterion to be applied.
Recommendations to users of monographs of active substances
Monographs give a specification for suitable quality of substances with impurity profiles corresponding to those taken into account during elaboration and/or revision of the monograph. It is the responsibility of the user of the substance to check that the monograph provides adequate control of impurities for a substance for pharmaceutical use from a given source, notably by using the procedure for certification of suitability of the monographs of the European Pharmacopoeia.
A monograph with a related substances test based on a quantitative method (such as liquid chromatography, gas chromatography and capillary electrophoresis) provides adequate control of impurities for a substance from a given source if impurities present in amounts above the applicable identification threshold are specified impurities mentioned in the Impurities section.
If the substance contains impurities other than those mentioned in the Impurities section, it has to be verified that these impurities are detectable by the method described in the monograph, otherwise a new method must be developed and revision of the monograph must be requested. Depending on the contents found and the limits proposed, the identification and/or the qualification of these impurities must be considered.
Where a single related substances test covers different impurity profiles, only impurities for the known profile from a single source need to be reported in the certificate of analysis unless the marketing authorisation holder uses active substances with different impurity profiles.
Identification of impurities (peak assignment)
Where a monograph has an individual limit for an impurity, it is often necessary to define means of identification, for example using a reference substance, a representative chromatogram or relative retention. The user of the substance may find it necessary to identify impurities other than those for which the monograph provides a means of identification, for example to check the suitability of the specification for a given impurity profile by comparison with the Impurities section. The European Pharmacopoeia does not provide reference substances, representative chromatograms or information on relative retentions for this purpose, unless prescribed in the monograph. Users will therefore have to apply the available scientific techniques for identification.
New impurities/Specified impurities above the specified limit
Where a new manufacturing process or change in an established process leads to the occurrence of a new impurity, it is necessary to apply the provisions of the general monograph on Substances for pharmaceutical use (2034) regarding identification and qualification and to verify the suitability of the monograph for control of the impurity. A certificate of suitability is a means for confirming for a substance from a given source that the new impurity is adequately controlled or the certificate contains a method for control with a defined acceptance criterion. In the latter case revision of the monograph will be initiated.
Where a new manufacturing process or change in an established process leads to the occurrence of a specified impurity above the specified limit, it is necessary to apply the provisions of the general monograph on Substances for pharmaceutical use (2034) regarding qualification.
Expression of acceptance criteria
The acceptance criteria for related substances are expressed in monographs either in terms of comparison of peak areas (comparative tests) or as numerical values.
Chromatographic methods
General chapter 2.2.46. Chromatographic separation techniques deals with various aspects of impurities control.
Information is available via the EDQM website on commercial names for columns and other reagents and equipment found suitable during monograph development, where this is considered useful.
GLOSSARY
Disregard limit
In chromatographic tests, the nominal content at or below which peaks/signals are not taken into account for calculating a sum of impurities. The numerical values for the disregard limit and the reporting threshold are usually the same.
Identification threshold
A limit above which an impurity is to be identified.
Identified impurity
An impurity for which structural characterisation has been achieved.
Impurity
Any component of a substance for pharmaceutical use that is not the chemical entity defined as the substance.
Nominal concentration
Concentration calculated on the basis of the concentration of the prescribed reference and taking account of the prescribed correction factor.
Other detectable impurities
Potential impurities with a defined structure that are known to be detected by the tests in a monograph but not known to be normally present above the identification threshold in substances used in medicinal products that have been authorised by the competent authorities of Parties to the Convention. They are unspecified impurities and are thus limited by a general acceptance criterion.
Potential impurity
An impurity that theoretically can arise during manufacture or storage. It may or may not actually appear in the substance. Where a potential impurity is known to be detected by the tests in a monograph but not known to be normally present in substances used in medicinal products that have been authorised by the competent authorities of Parties to the Convention, it will be included in the Impurities section under Other detectable impurities for information.
Qualification
The process of acquiring and evaluating data that establishes the biological safety of an individual impurity or a given impurity profile at the level(s) specified.
Qualification threshold
A limit above which an impurity is to be qualified.
Related substances
Title used in monographs for general tests for organic impurities.
Reporting threshold
A limit above which an impurity is to be reported. Synonym: reporting level.
Specified impurity
An impurity that is individually listed and limited with a specific acceptance criterion in a monograph. A specified impurity can be either identified or unidentified.
Unidentified impurity
An impurity for which a structural characterisation has not been achieved and that is defined solely by qualitative analytical properties (for example, relative retention).
Unspecified impurity
An impurity that is limited by a general acceptance criterion and not individually listed with its own specific acceptance criterion.