Purified Water
Ph Eur
DEFINITION
Water for the preparation of medicines other than those that are required to be both sterile and apyrogenic, unless otherwise justified and authorised.
Purified water in bulk
PRODUCTION
Purified water in bulk is prepared by distillation, by ion exchange, by reverse osmosis or by any other suitable method from water that complies with the regulations on water intended for human consumption laid down by the competent authority.
Purified water in bulk is stored and distributed in conditions designed to prevent growth of micro-organisms and to avoid any other contamination.
Microbiological monitoring
During production and subsequent storage, appropriate measures are taken to ensure that the microbial count is adequately controlled and monitored. Appropriate alert and action levels are set so as to detect adverse trends. Under normal conditions, an appropriate action level is a microbial count of 100 CFU/mL, determined by filtration through a membrane with a nominal pore size not greater than 0.45 µm, using R2A agar and incubating at 30-35 °C for not less than 5 days. The size of the sample is to be chosen in relation to the expected result.
R2A agar
Adjust the pH so that after sterilisation it is 7.2 ± 0.2. Sterilise by heating in an autoclave at 121 °C for 15 min.
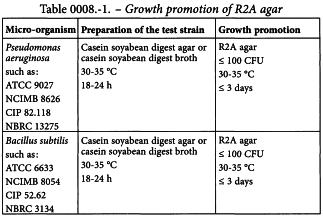
Growth promotion of R2A agar
Total organic carbon or oxidisable substances
Carry out the test for total organic carbon (2.2.44) with a limit of 0.5 mg/L or alternatively the following test for oxidisable substances: to 100 mL add 10 mL of dilute sulfuric acid R and 0.1 mL of 0.02 M potassium permanganate and boil for 5 min; the solution remains faintly pink.
Conductivity
Determine the conductivity off-line or in-line under the following conditions.
Equipment
Conductometer Accuracy of 0.1 µS⋅cm-1 or better at the lowest range.
Conductometer calibration Calibration is carried out for each range of measurement to be used, after disconnection of the conductivity cell, using certified precision resistors or equivalent devices with an uncertainty not greater than 0.1 per cent of the certified value.
If in-line conductivity cells cannot be dismantled, system calibration may be performed against a calibrated conductivity-measuring instrument with a conductivity cell placed close to the cell to be calibrated in the water flow.
Temperature measurement Tolerance ± 2 °C.
PROCEDURE
Measure the conductivity without temperature compensation, recording simultaneously the temperature. Temperature-compensated measurement may be performed after suitable validation.
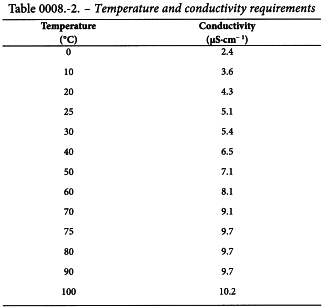
The water to be examined meets the requirements if the measured conductivity at the recorded temperature is not greater than the value in Table 0008.-2.
For temperatures not listed in Table 0008.-2, calculate the maximal permitted conductivity by interpolation between the next lower and next higher data points in the table.
Heavy metals
If purified water in bulk complies with the requirement for conductivity prescribed for Water for injections (0169) in bulk, it is not necessary to carry out the test for heavy metals prescribed below.
CHARACTERS
Appearance
Clear and colourless liquid.
TESTS
Nitrates
Maximum 0.2 ppm.
Place 5 mL in a test-tube immersed in iced water, add 0.4 mL of a 100 g/L solution of potassium chloride R, 0.1 mL of diphenylamine solution R and, dropwise with shaking, 5 mL of nitrogen-free sulfuric acid R. Transfer the tube to a water-bath at 50 °C. After 15 min, any blue colour in the solution is not more intense than that in a reference solution prepared at the same time in the same manner using a mixture of 4.5 mL of nitrate-free water R and 0.5 mL of nitrate standard solution (2 ppm NO3) R.
Aluminium (2.4.17)
Maximum 10 ppb, if intended for use in the manufacture of dialysis solutions.
Prescribed solution To 400 mL of the water to be examined add 10 mL of acetate buffer solution pH 6.0 R and 100 mL of distilled water R.
Reference solution Mix 2 mL of aluminium standard solution (2 ppm Al) R, 10 mL of acetate buffer solution pH 6.0 R and 98 mL of distilled water R.
Blank solution Mix 10 mL of acetate buffer solution pH 6.0 R and 100 mL of distilled water R.
Heavy metals (2.4.8)
Maximum 0.1 ppm.
To 200 mL add 0.15 mL of 0.1 M nitric acid and heat in a glass evaporating dish on a water-bath until the volume is reduced to 20 mL. 12 mL of the concentrated solution complies with test A. Prepare the reference solution using 10 mL of lead standard solution (1 ppm Pb) R and adding 0.075 mL of 0.1 M nitric acid. Prepare the blank solution adding 0.075 mL of 0.1 M nitric acid.
Bacterial endotoxins (2.6.14)
Less than 0.25 IU/mL, if intended for use in the manufacture of dialysis solutions without a further appropriate procedure for removal of bacterial endotoxins.
LABELLING
The label states, where applicable, that the substance is suitable for use in the manufacture of dialysis solutions.
Purified water in containers
DEFINITION
Purified water in bulk that has been filled and stored in conditions designed to assure the required microbiological quality. It is free from any added substances.
CHARACTERS
Appearance
Clear and colourless liquid.
TESTS
It complies with the tests prescribed in the section on Purified water in bulk and with the following additional tests.
Acidity or alkalinity
To 10 mL, freshly boiled and cooled in a borosilicate glass flask, add 0.05 mL of methyl red solution R. The solution is not coloured red.
To 10 mL add 0.1 mL of bromothymol blue solution R1. The solution is not coloured blue.
Oxidisable substances
To 100 mL add 10 mL of dilute sulfuric acid R and 0.1 mL of 0.02 M potassium permanganate and boil for 5 min. The solution remains faintly pink.
Chlorides
To 10 mL add 1 mL of dilute nitric acid R and 0.2 mL of silver nitrate solution R2. The solution shows no change in appearance for at least 15 min.
Sulfates
To 10 mL add 0.1 mL of dilute hydrochloric acid R and 0.1 mL of barium chloride solution R1. The solution shows no change in appearance for at least 1 h.
Ammonium
Maximum 0.2 ppm.
To 20 mL add 1 mL of alkaline potassium tetraiodomercurate solution R. After 5 min, examine the solution down the vertical axis of the tube. The solution is not more intensely coloured than a standard prepared at the same time by adding 1 mL of alkaline potassium tetraiodomercurate solution R to a mixture of 4 mL of ammonium standard solution (1 ppm NH4) R and 16 mL of ammonium-free water R.
Calcium and magnesium
To 100 mL add 2 mL of ammonium chloride buffer solution pH 10.0 R, 50 mg of mordant black 11 triturate R and 0.5 mL of 0.01 M sodium edetate. A pure blue colour is produced.
Residue on evaporation
Maximum 0.001 per cent.
Evaporate 100 mL to dryness on a water-bath and dry in an oven at 100-105 °C. The residue weighs a maximum of 1 mg.
Microbial contamination
TAMC: acceptance criterion 102 CFU/mL (2.6.12). Use casein soya bean digest agar.
LABELLING
The label states, where applicable, that the substance is suitable for use in the manufacture of dialysis solutions.
Ph Eur