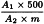Pillules for Homoeopathic Preparations
Ph Eur
DEFINITION
Solid preparations obtained from sucrose, lactose or other suitable excipients. They possess a suitable mechanical strength to resist handling without crumbling or breaking. They are intended for impregnation or coating with one or more homoeopathic preparations. The impregnated pillules comply with the requirements of the monograph Homoeopathic pillules, impregnated (2079). The coated pillules comply with the requirements of the monograph Homoeopathic pillules, coated (2786).
PRODUCTION
In the manufacture, packaging, storage and distribution of pillules for homoeopathic preparations, suitable measures are taken to ensure their microbiological quality; recommendations on this aspect are provided in general chapter 5.1.4. Microbiological quality of non-sterile pharmaceutical preparations and substances for pharmaceutical use.
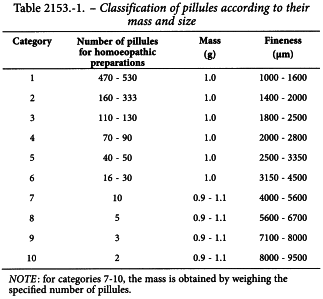
If a system of sizing is used, the indications in Table 2153.-1 are used.
CHARACTERS
Appearance
White or almost white spheroids.
Solubility
Usually freely soluble in water.
IDENTIFICATION
The excipients used for the manufacture of pillules for homoeopathic preparations are identified by one or more suitable test(s).
TESTS
If the test for fineness is carried out, the test for uniformity of mass need not be carried out, and vice versa.
Uniformity of mass
Carry out the test using 20 pillules to constitute 1 unit. Weigh individually 20 units taken at random and determine the individual and average masses. Not more than 2 of the individual masses deviate from the average mass by more than 10 per cent and none deviate by more than 20 per cent.
Fineness (2.9.35)
Not less than 90 per cent m/m of the pillules are between the lower and upper limits of the corresponding category as indicated in Table 2153.-1.
Uniformity of impregnation
None of the individual values deviate by more than 10 per cent from the average of 10 determinations.
Methylene blue impregnation solutionUse a freshly prepared solution. Dissolve 1.000 g of methylene blue R in 50 mL of ethanol (70 per cent V/V) R and dilute to 1000.0 mL with the same solvent.
Test solution Impregnate a suitable quantity of pillules for homoeopathic preparations with a suitable quantity of the methylene blue impregnation solution, to achieve a content of 10 µL of impregnation solution per gram of pillules. Dissolve 5.000 g (m g) of these impregnated pillules in water R and dilute to 25.0 mL with the same solvent.
Reference solution Dilute 1.0 mL of the methylene blue impregnation solution to 100.0 mL with water R. To 5.0 mL of this solution add 5.000 g of pillules for homoeopathic preparations, dissolve in water R and dilute to 25.0 mL with the same solvent.
Measure the absorbance (2.2.25) of the test solution and the reference solution at 665 nm. Calculate the percentage of impregnation of the pillules for homoeopathic preparations using the following expression:
A1 | = | absorbance of the test solution; |
A2 | = | absorbance of the reference solution; |
m | = | mass of the impregnated pillules used to prepare the test solution, in grams. |
Carry out 10 individual determinations.
Microbial contamination
TAMC: acceptance criterion 102 CFU/g (2.6.12).
TYMC: acceptance criterion 101 CFU/g (2.6.12).
Absence of Staphylococcus aureus (2.6.13).
Absence of Pseudomonas aeruginosa (2.6.13).
LABELLING
Ph Eur