Sumatriptan
Action and use
Serotonin 5HT1 receptor agonist; treatment of migraine.
Preparation
Definition
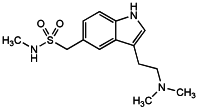
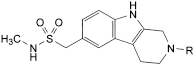
Sumatriptan is 3-(2-dimethylaminoethyl)indol-5-yl-N-methylmethanesulfonamide. It contains not less than 97.5% and not more than 102.0% of C14H21N3O2S, calculated with reference to the anhydrous substance.
Characteristics
A white to pale yellow powder. Very slightly soluble in water.
Identification
The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of sumatriptan (RS 414).
Tests
The total impurity content in the test for Impurities A and H and the test for Related substances is not greater than 1.5%.
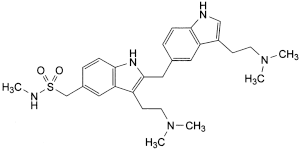
Impurities A and H
Carry out the method for liquid chromatography, Appendix III D. Prepare the solutions in a mixture containing 3 volumes of 0.025M sodium dihydrogen orthophosphate, the pH of which has been adjusted to 6.5, and 1 volume of acetonitrile (solution A).
10 volumes of 10m ammonium acetate and 90 volumes of methanol.
The test is not valid unless the chromatogram obtained with solution (3) resembles that supplied with sumatriptan for system suitability EPCRS and the resolution factor between impurity A and sumatriptan is at least 1.5.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity A is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.6%; taking into account the correction factor of 0.6 for impurity A);
the area of any peak due to impurity H is not greater than 0.3 times the area of the principal peak in the chromatogram obtained with solution (2) (0.3%).
Related substances
Carry out the method for liquid chromatography, Appendix III D. Prepare the solutions in a mixture containing 3 volumes of 0.025M sodium dihydrogen orthophosphate, the pH of which has been adjusted to 6.5, and 1 volume of acetonitrile (solution A).
Mix 25 volumes of acetonitrile with 75 volumes of a solution containing 0.97 g of dibutylamine, 0.735 g of orthophosphoric acid and 2.93 g of sodium dihydrogen orthophosphate in 750 mL water, adjust to pH 6.5 with 10m sodium hydroxide and dilute to 1000 mL with water.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks due to impurity C and sumatriptan is at least 1.5.
In the chromatogram obtained with solution (1):
the areas of any peaks corresponding to impurities B, C and D are not greater than 5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5% of each);
the area of any peak corresponding to impurity E is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.1%);
the area of any other secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.1%).
Disregard any peak with an area less than 0.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.05%).
Water
Not more than 1.0% w/w, Appendix IX C. Use 1.0 g.
Assay
Carry out the method for liquid chromatography, Appendix III D. Prepare solutions (1) and (2) in a mixture containing 3 volumes of 0.025M sodium dihydrogen orthophosphate, the pH of which has been adjusted to 6.5, and 1 volume of acetonitrile (solution A).
The chromatographic conditions described under Related substances may be used.
The Assay is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks due to sumatriptan and impurity C is at least 1.5.
Calculate the content of C14H21N3O2S using the declared content of C14H21N3O2S,C4H6O4 in sumatriptan succinate BPCRS. Each 1 mg of C14H21N3O2S is equivalent to 1.4 mg of C14H21N3O2S,C4H6O4.
Storage
Sumatriptan should be protected from light.