Water for Preparation of Extracts
Ph Eur
DEFINITION
Water intended for the preparation of Herbal drug extracts (0765) complies with the sections Purified water in bulk or Purified water in containers in the monograph Purified water (0008), or is water intended for human consumption of a quality equivalent to that defined in Directive 98/83/EC which is monitored according to the Production section described below.
PRODUCTION
When water intended for human consumption is used as water for preparation of extracts it is a clear, colourless liquid. It is stored (where necessary) and distributed under conditions designed to prevent growth of micro-organisms and to avoid other contamination.
For monitoring purposes, the following tests are carried out at regular intervals to demonstrate consistency in the quality of the water used for the preparation of extracts.
Conductivity (2.2.38)
Maximum 2500 µS⋅cm-1, measured at 20 °C.
Nitrate
Liquid chromatography (2.2.29).
Test solution The substance to be examined.
Reference solution Dissolve 0.163 g of potassium nitrate R and 0.149 g of potassium bromide R in water R and dilute to 100.0 mL with the same solvent. Dilute 5.0 mL of the solution to 100.0 mL with water R.
Mobile phase Dissolve 0.265 g of anhydrous sodium carbonate R and 0.210 g of sodium hydrogen carbonate R in water R and dilute to 1000.0 mL with the same solvent.
Flow rate 1.2 mL/min.
Detection Conductivity detector, using a self-regenerating anion suppressor.
Injection 25 µL.
Run time Twice the retention time of nitrate.
Relative retention With reference to nitrate (retention time = about 7 min): bromide = about 0.9.
Microbiological monitoring
Appropriate measures are taken to ensure that the microbial count is adequately controlled and monitored. Appropriate alert and action levels are set so as to detect adverse trends.
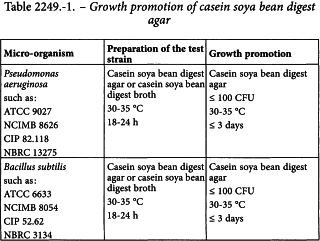
Under normal conditions, an appropriate action level is a microbial count of 100 CFU/mL, determined by filtration through a membrane with a nominal pore size not greater than 0.45 µm, using casein soya bean digest agar and incubating at 30-35 °C for not less than 5 days.
The size of the sample is to be chosen in relation to the expected result.
Adjust the pH so that after sterilisation it is 7.3 ± 0.2. Sterilise in an autoclave using a validated cycle.
Growth promotion of casein soya bean digest agar
Ph Eur