Appendix III M. Glycan Analysis of Glycoproteins
1 Introduction
Glycan analysis is a test to analyse glycan moieties of glycoproteins. It may involve:
Monosaccharide analysis may complement information obtained by glycan analysis.
Glycosylation can play a predominant role in determining the function, pharmacokinetics, pharmacodynamics, stability, and immunogenicity of biotherapeutics. Glycosylation, unlike transcription, is a non-template-driven enzymatic modification process that results in glycan heterogeneity. The manufacturing procedure also has an influence on glycan heterogeneity. Glycoprotein glycan analysis may therefore be an important test to identify variations in the glycosylation pattern of the glycoprotein and/or monitor the consistency of the glycosylation pattern during production.
Glycan analysis can be a comparative procedure, because the information obtained, compared to a similarly treated reference substance, confirms product consistency.
This chapter provides approaches used for glycoprotein glycan analysis and requirements for the application of methods and validation of methods.
Glycan analysis is not a single general method, but involves the application of specific procedures and the development of specific glycan maps for each unique glycoprotein. Specific procedures are therefore indicated in relevant specific monographs.
1-1 Protein glycosylation
There are 3 main types of enzymatic glycosylation found in proteins:
Non-enzymatic additions, also known as glycation, can occur when proteins are incubated with reducing sugars.
This chapter describes analytical methods for the N- and O-linked glycosylations, which are the most commonly found in glycoprotein medicinal products.
1-2 Heterogeneity of the protein glycosylation
Different levels of glycan heterogeneity can appear during the production of glycoproteins. This heterogeneity may result from variations:
This heterogeneity in glycosylation results in a set of glycoforms for one specific glycoprotein. These variations arise because, unlike transcription and translation, glycosylation is a non-template post-translational modification process. The glycosylation pattern at a given site depends on many factors including the cell-specific and/or growth-dependent availability of glycosyltransferases and exo-glycosidases found in the Golgi apparatus and endoplasmic reticulum. Protein glycosylation is also influenced by the protein structure, the production process, the host-vector expression system and the cell culture conditions.
2 Glycan analysis procedures
Heterogeneity in glycosylation can be assessed by 4 distinct and complementary approaches:
The present section provides methods and general requirements used for glycan analysis of glycoproteins containing N- and O-linked glycans.
Glycan analysis is usually a multistep process. There are numerous methodologies for glycan analysis. This variety is a consequence of the diversity and complexity of glycan structures, of the available technologies and detection systems, and of the wide range of approaches depending on the level of information required.
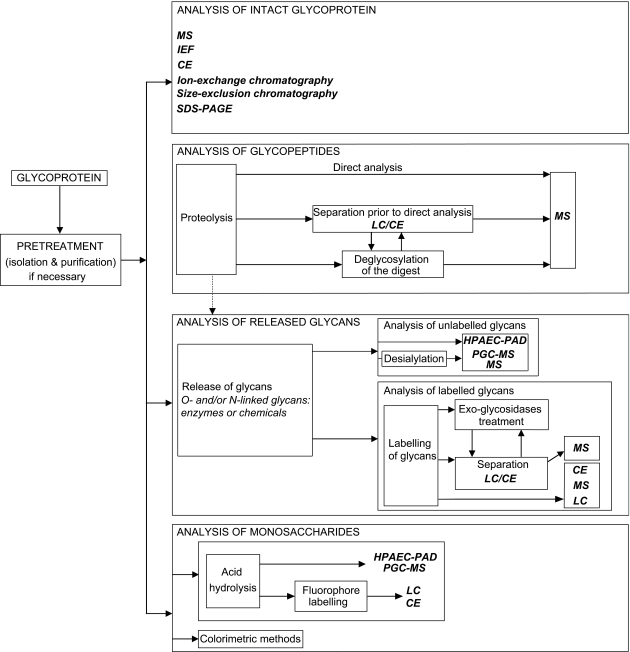
Figure 2.2.59.-1 provides an overview of glycan analysis analytical procedures that can be employed to apply the chosen approach(es). Many variations of the same techniques and conditions are available depending on the glycan structures and origin.
| CE: | Capillary electrophoresis |
| HPAEC-PAD: | High-pH anion-exchange chromatography with pulsed amperometric detection |
| IEF: | Isoelectric focusing |
| LC: | |
| MS: | |
| PGC: | Porous graphite chromatography |
| SDS-PAGE: | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
Isolation and purification
Isolation and purification may be necessary for analysis of bulk drug substances or dosage forms containing interfering excipients, and, when required, will be described in the specific monograph.
2-1 Analysis of intact glycoprotein
Analysis of the intact glycoprotein provides information on the overall pattern of glycosylation of the glycoprotein.
This approach provides limited information when the molecule is large and contains multiple glycosylation sites.
Methods such as capillary electrophoresis (CE) (2.2.47) and mass spectrometry (MS) (2.2.43) can be used. Size-based techniques, such as size-exclusion chromatography (2.2.30) and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (2.2.31), may provide information on the glycosylation status of a protein. If the degree of sialylation significantly contributes to the biological activity of the glycoprotein, ion-exchange chromatography (2.2.46), isoelectric focusing (IEF) (2.2.54) or CE (2.2.47) may be performed to monitor sialylation. The technique must be chosen according to its suitability to provide a reliable correlation between the degree of sialylation and the bioactivity of the product.
2-2 Analysis of glycopeptides
Analysis of glycopeptides provides information on site-specific glycosylation properties, on the degree of occupancy, and on the oligosaccharide structures. It involves proteolytic digestion of the glycoprotein. Approaches to site-specific cleavage of the protein backbone are given in general chapter 2.2.55. Peptide mapping.
After proteolysis of the glycoprotein, the following approaches can be chosen.
Direct analysis by MS ()2.2.43 Care should be taken that the glycopeptide signal is not suppressed due to the presence of other peptides, where glycopeptides represent a minor portion of the total peptide mixture and where signal intensities are lower than those of non-glycosylated peptides.
Separation prior to analysis by MS This additional step overcomes the problems raised above. Enrichment or fractionation techniques can be used either in parallel with or sequentially to direct analysis. Separation techniques such as liquid chromatography (LC) (2.2.29) and CE (2.2.47) are suitable. These techniques may be interfaced with MS to allow online MS measurements.
Deglycosylation of the glycopeptides Identification of the different glycosylation sites of a glycoprotein is made possible by comparing peptide maps obtained by proteolytic digestion of the intact glycoprotein to those obtained when the glycoprotein is deglycosylated previously or following proteolytic digestion. The peptide mass gives information about the glycosylation sites and by calculating the mass difference between the intact glycopeptide and the deglycosylated glycopeptide, it is possible to obtain information about the attached glycans concerning composition and heterogeneity. Approaches to deglycosylation of the protein backbone are given in section 2-3-1. A separation step can be performed after or before deglycosylation.
2-3 Analysis of released glycans
Analysis of released glycans provides a convenient way to obtain information on the various populations of glycans present on the protein (bi-, tri-, and tetra-antennary profile). The degree of sialylation can also be addressed at this stage. Depending on the chosen method, prior derivatisation/labelling may be needed to allow the detection of the glycans.
Analysis of released glycans generally involves the release and purification of glycans from the reaction mixture, followed by the labelling/derivatisation of the glycans, where needed; the glycans are then profiled (fractionation or separation).
2-3-1 Release of glycans
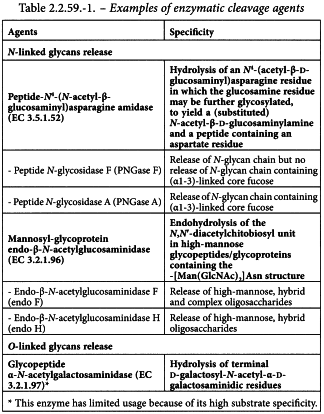
The selection of the approach used for the release of glycans will depend on the glycoprotein under test. The cleavage agent to be employed is chosen according to the type of cleavage needed and level of information required. Enzymatic or chemical cleavage may be used. Table 2.2.59.-1 gives a non-exhaustive list of enzymatic cleavage agents and their specificity.
Digestion efficiency is generally dependent on the accessibility of the glycans on the protein and hence the protein can be denatured to maximise glycosylation site exposure, unless it is desirable to distinguish between surface and buried glycans.
Chemical cleavage agents might also be used, using for example hydrazine or alkaline borohydride for β-elimination.
2-3-2 Analysis of glycans
Released glycans can be analysed or profiled by chromatographic, electrophoretic and mass spectrometric techniques, and in general by a combination of these techniques. The choice of the method can be grouped according to the nature of the glycans and level of information required.
Analysis of glycans provides information on the various populations of glycans present on the protein (high-mannose, hybrid, complex). Information on the relative amounts of branched structures might be obtained by analysis of desialylated glycans.
A separation step may be required. It implies the use of LC (2.2.29) and CE (2.2.47) as intermediate techniques. LC (2.2.29) can be used preparatively with individual fractions being collected (usually labelling is required) or can be directly coupled to MS (2.2.43).
2-3-2-1 Analysis of unlabelled glycans
Native glycans can be analysed by high-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD), porous graphite chromatography (PGC) and MS (2.2.43).
HPAEC-PAD has high sensitivity and can also separate some linkage isomers. Response factors of the different signals are not equal for the different oligosaccharide structures. Absolute quantification of the glycan is not possible unless an oligosaccharide reference library is available. Quantification can be obtained by comparison with a well-characterised reference standard of the substance being tested, or by relating the peak area of each glycan to the total peak area of all glycans in the map.
PGC can also be used to separate native glycans because of its higher selectivity compared to the conventional non-polar columns. A PGC-electrospray-ionisation-MS approach can be applied for direct glycan analysis.
2-3-2-2 Analysis of labelled glycans
Labelling of glycans
The type of derivatisation carried out will depend on the method used to detect glycans: UV or fluorescent.
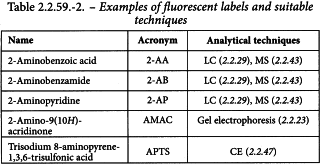
Derivatisation with fluorescent labels is the most commonly used technique for labelling glycans at their reducing end by reductive amination. One label can be attached to every single mono- and oligo-saccharide, allowing the determination of molar quantities. Table 2.2.59.-2 gives a non-exhaustive list of commonly used fluorescent labels and suitable analytical techniques.
Permethylation of glycans may also be used when MS (2.2.43) is used alone for detection. It is based on the methylation of the oligosaccharides.
Analysis of labelled glycans
Labelled glycans can be analysed by analytical techniques such as LC (2.2.29), CE (2.2.47) and MS (2.2.43).
According to the separation properties of the glycans, glycans can be profiled and quantified by several LC (2.2.29) systems using an appropriate label: reversed-phase (separation by hydrophobicity), normal-phase (separation by size), and anion-exchange (separation by charge) LC.
2-4 Monosaccharide analysis
Monosaccharide analysis provides information on the monosaccharide composition of a glycoprotein. Analysis of monosaccharides can be performed using either colorimetric or separation methods.
2-4-1 Colorimetric methods
The colorimetric methods, which are based on chemical staining, provide information on the quantity of specific classes of sugars such as sialic acids, neutral sugars and hexosamines.
2-4-2 Separation methods
The separation methods generate quantitative information on the overall monosaccharide composition. The methods require acid hydrolysis pre-treatment of the oligosaccharide chains of the intact glycoprotein or released glycans, prior to analysis. To release sialic acids, mild acid hydrolysis or enzymatic treatment is employed. The hydrolysis step is a significant source of variability and may require product-specific validation.
Methods for separation and quantification of monosaccharides include:
3 evaluation and analysis of data
Data obtained from analytical methods for the analysis of glycans can be analysed and evaluated for 3 different purposes:
Specific considerations with respect to reference standards and method development of each level of analysis are set out in sections 4 and 5 respectively.
3-1 Confirmation of identity of individual structures or families of structures
The analytical target for a glycan analysis method may be an individual monosaccharide (e.g. sialic acid, fucose), a defined oligosaccharide structure (e.g. tetra-sialylated, tetra-antennary glycan) or a family of structures sharing a common analytical feature (e.g. tetra-sialylated glycans, tri-antennary glycans, glycoprotein isoforms with the same charge). Confirmation of the identity of the analytical target is an essential step in the analysis and evaluation of data, and can be achieved absolutely, by verification of molecular structure, or comparatively, by comparison with an appropriate reference standard.
3-1-1 Absolute confirmation of identity
Absolute confirmation of the identity of glycan structures is typically achieved during product development, and should not necessarily be the target of routine analysis. Identity of the analytical target will be assigned by reference to a known molecular property of the molecule. Such absolute identification of individual structures can require multi-step approaches using enzymatic and chemical reactions, separation techniques and online or offline detection methods, and will most commonly use the charge-to-mass ratio of a molecular ion, determined using a suitable mass spectrometric method as the final basis for structure assignment.
3-1-2 Comparative confirmation of identity
During routine application of the analytical method, the identity of the analytical target may be confirmed by comparison with process or system suitability reference standards. These may be generated from known, well-characterised glycoproteins, which may be of the same general class as the product being tested (e.g. fetuin for complex N-linked glycoproteins), or may be derived from a well-characterised batch of the product being tested, which has been established as a reference standard. The following considerations apply to comparative assignment of structural identity:
3-2 Confirmation of compliance of the substance being tested with qualitative requirements
At this level of evaluation, the analytical results obtained with the product being tested are evaluated to demonstrate compliance with specifications. Typically this is achieved by comparison with data obtained in parallel using a reference standard of the substance being tested. In evaluating the data it is necessary:
3-3 Confirmation of compliance of the substance being tested with quantitative requirements
3-3-1 Quantitative measurement of analyte levels and expression of results
In some cases, e.g. measurement of sialic acid or other monosaccharides, data can be expressed in order to obtain a molar ratio of sialic acid to glycoprotein. Data is calculated by reference to a reference standard for sialic acid and to a validated method of protein determination. Either the internal or external standard method may be used (see general chapter 2.2.46. Chromatographic separation techniques).
3-3-2 Quantitative expressions of separation profile
Profiles or distribution patterns may be expressed numerically in a number of ways, including the normalisation procedure; the percentage content of each analytical target, e.g. glycan entity, is calculated by determining the response of the glycan entity as a percentage of the total response of all the entities, excluding those due to solvents or any added reagents, and those below the disregard limit. In addition, numerical expressions such as the Z number, which are method- and product-specific and defined in specific monographs, can be used.
4 Reference standards
Reference standards for glycan analysis serve 2 functions: the verification of the suitability of the system and the confirmation that the article under test complies with specified requirements.
The reference standards used for system suitability may be:
The reference standard used for compliance of the glycoprotein under test is a preparation of the substances being tested. It is noted that glycan analysis procedures described in specific monographs prescribe the use of a reference standard for the substance being tested and for which the glycan analysis procedure has been validated.
5 POINTS TO CONSIDER IN METHOD DEVELOPMENT
This section provides means for measuring the overall performance of the method during development. The extent of method development and analytical validation is selected on the basis of their suitability for a specific product. Depending on the chosen approach, several steps are necessary for glycan analysis, for example:
Protein isolation and purification
Isolation and purification of the glycoprotein from its matrix may be necessary to remove all interfering substances (e.g. excipients, salts) and, when required, will be specified in the specific monograph. This must be performed in a reproducible manner in order to guarantee a quantitative recovery of the protein.
Release and isolation of oligosaccharides
The approach chosen for the release of glycans will depend on the protein under test and will be based on the types of glycosylation, i.e. N- or O-linked glycosylation. Non-compendial approaches available for the release of glycans must be optimised in order to ascertain a quantitative profiling of all glycan entities. Factors that impact cleavage efficiency, such as enzyme-to-protein concentration ratio, temperature, reaction time course, and denaturation of protein prior to digestion, must be optimised.
It is noteworthy that the enzymatic/chemical reaction must not alter the glycan composition, e.g. not destroy sialic acid residues. Where there is more than one glycosylation site, the enzymatic treatment should proportionally release all oligosaccharide moieties attached to the protein, independent of their structure and their individual position in the protein. Reproducible recovery of all glycan entities from the reaction mixture must be confirmed.
Derivatisation of released glycans
Derivatisation is usually carried out according to non-compendial protocols. Therefore, the reproducible derivatisation of all glycan entities must be verified. This may be achieved through optimisation of the reaction conditions such as amount of the derivatisation reagent, reaction temperature and time. The derivatisation reaction must not change the glycan composition, e.g. not destroy sialic acid residues.
Separation, identification and system suitability
The methods employed for glycan analysis must be capable of detecting and separating different glycan moieties to ascertain a reliable identification and quantification.
The acceptance criteria for system suitability, which also cover glycan cleavage, recovery and analysis, depend on the critical test parameters that affect the outcome of the result.
A comparison between the glycan map of the substance under test and that of a reference substance, being treated in the same conditions, is an indicator to evaluate the performance of the analytical procedure. In order to further confirm the obtained results, the analyses may be repeated with an orthogonal method. The use of a reference standard (e.g. reference substance of the product being examined, system suitability glycan marker) is essential in the establishment of system suitability parameters and validation of the analytical procedure.
Reproducibility of quantitative expression (e.g. Z number estimation) of glycan profiles must be verified.
Determination of site occupancy based on relative quantities of glycosylated and non-glycosylated peptides
Where site occupancy is estimated by comparison of glycosylated and non-glycosylated peptides from an enzymatically digested glycoprotein, reproducible cleavage of both forms of the peptide must be demonstrated.
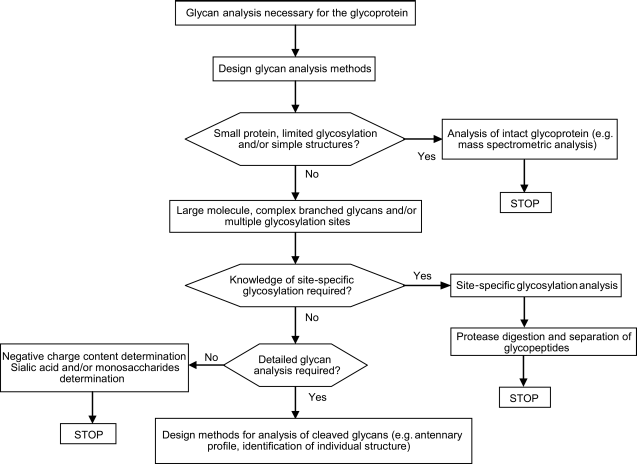
6 Glycan analysis decision-making framework
This decision-making framework is given for information and does not constitute a mandatory part of the European Pharmacopoeia.
The choice of procedures used to analyse glycans is established according to the level of information required to ensure the quality of the glycoprotein and is set up during the development phase of the product.
Figure 2.2.59.-2 provides guidance in the choice of methods to be used when glycan analysis is required.