Appendix XIX E. Rubber Closures for Containers for Aqueous Parenteral Preparations
Rubber closures for containers for aqueous parenteral preparations, for powders and for freeze-dried powders are made of materials obtained by vulcanisation (cross-linking), using appropriate additives, of macromolecular organic substances (elastomers). The elastomers are produced from natural or synthetic substances by polymerisation. The choice of the principal components and of the various additives (for example, vulcanisers, accelerators, stabilisers, pigments) depends on the properties required for the finished article. The specifications do not apply to closures made from silicone elastomer (which are dealt with in chapter 3.1.9. Silicone elastomer for closures and tubing), to laminated closures or to lacquered closures.
Rubber closures may be classified in 2 types: type I closures meet the strictest requirements and are preferred; type II closures have mechanical properties suitable for special uses (for example, multiple piercing) and cannot meet requirements as severe as for type I closures because of their chemical composition.
The closures chosen for use with a particular preparation are such that:
The closures are compatible with the preparation for which they are used throughout its period of validity.
The manufacturer of the preparation must obtain from the supplier an assurance that the composition of the closure does not vary and that it is identical to that of the closure used during compatibility testing. If the supplier informs the manufacturer of the preparation that changes have been made to the composition, compatibility testing must be repeated, totally or partly, depending on the nature of the changes.
The closures are washed and may be sterilised before use.
CHARACTERS
Rubber closures are elastic. They are translucent or opaque and have no characteristic colour, the latter depending on the additives used. They are practically insoluble in tetrahydrofuran, in which, however, a considerable reversible swelling may occur. They are homogeneous and practically free from flash and adventitious materials (for example, fibres, foreign particles, waste rubber).
Identification of the type of rubber used for the closures is not within the scope of this specification. The identification tests given below distinguish between closures made from rubber and those made from silicone elastomer and plastic materials but do not differentiate all types of rubber. Other identity tests may be carried out with the aim of detecting differences in a batch compared with the closures used for compatibility testing. One or more of the following analytical methods may be applied for this purpose: determination of relative density, determination of sulfated ash, determination of sulfur content, thin-layer chromatography carried out on an extract, ultraviolet absorption spectrophotometry of an extract, infrared absorption spectrophotometry of a pyrolysate or attenuated total reflectance (ATR).
IDENTIFICATION
Examine by attenuated total reflectance (ATR). The spectrum obtained is identical to the spectrum obtained with the type sample. If necessary, cut the sample along an appropriate axis, examine the cut surface and compare the spectrum with that obtained with the type sample prepared in the same way.
If direct ATR measurement on the surface is not feasible (mainly rubber closures filled with carbon black), heat 1-2 g in a heat-resistant test-tube over an open flame to dry the sample and continue heating until pyrolysate vapours are condensed near the top edge of the test-tube. Examine the pyrolysate of the sample by ATR and compare the spectrum with that obtained with the pyrolysate of the type sample.
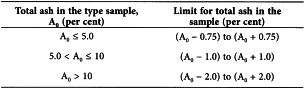
Determine the percentage content of total ash in the sample to be examined and compare with the percentage content of total ash in the type sample (A0). The total ash content falls within the following ranges depending on the total ash content of the type sample.
In addition to the use of platinum and silica crucibles described in general chapter 2.4.16, porcelain crucibles may be used. The sample may be ignited using a microwave oven instead of a muffle furnace.
TESTS
The samples to be analysed may be washed and sterilised before use.
Solution S
Place a number of uncut closures with a total surface area of about 100 cm2 in a suitable glass container, cover with water R, boil for 5 min and rinse 5 times with cold water R. Place the washed closures in a wide-necked flask (type I glass, 3.2.1), add 200 mL of water R and weigh. Cover the mouth of the flask with a borosilicate-glass beaker. Heat in an autoclave so that a temperature of 121 ± 2 °C is reached within 20-30 min and maintain at this temperature for 30 min. Cool to room temperature over about 30 min. Make up to the original mass with water R. Shake and decant the solution immediately. Shake solution S before each test. If using a tightly closed flask (type I glass, 3.2.1) with an inert closure instead of a wide-necked flask covered with a borosilicate-glass beaker, it is not necessary to make up to the original mass.
Blank solution Prepare a blank solution in the same manner using 200 mL of water R.
Appearance of solution S
For type I closures, solution S is not more opalescent than reference suspension II (2.2.1) and for type II closures, solution S is not more opalescent than reference suspension III. Solution S is not more intensely coloured than reference solution GY5 (2.2.2, Method II).
Acidity or alkalinity
To 20 mL of solution S add 0.1 mL of bromothymol blue solution R1. Not more than 0.3 mL of 0.01 M sodium hydroxide or 0.8 mL of 0.01 M hydrochloric acid is required to change the colour of the indicator to blue or yellow, respectively.
Absorbance
Carry out the test within 5 h of preparation of solution S Filter solution S through a membrane filter (nominal pore size 0.45 µm), rejecting the first few millilitres of filtrate. Measure the absorbance (2.2.25) of the filtrate at wavelengths from 220-360 nm using the blank (see solution S) as compensation liquid. At these wavelengths, the absorbance does not exceed 0.2 for type I closures or 4.0 for type II closures. If necessary, dilute the filtrate before measurement of the absorbance and correct the result for the dilution.
Reducing substances
Carry out the test within 4 h of preparation of solution S To 20.0 mL of solution S add 1 mL of dilute sulfuric acid R and 20.0 mL of 0.002 M potassium permanganate. Boil for 3 min. Cool. Add 1 g of potassium iodide R and titrate immediately with 0.01 M sodium thiosulfate, using 0.25 mL of starch solution R as indicator. Carry out a titration using 20.0 mL of the blank. The difference between the titration volumes is not greater than 3.0 mL for type I closures and 7.0 mL for type II closures.
Ammonium (2.4.1, Method A)
Maximum 2 ppm.
Dilute 5 mL of solution S to 14 mL with water R.
Extractable zinc
Maximum of 5 µg of extractable Zn per millilitre of solution S.
Atomic absorption spectrometry (2.2.23, Method I).
Test solution Dilute 10.0 mL of solution S to 100 mL with 0.1 M hydrochloric acid.
Reference solutions Prepare the reference solutions using zinc standard solution (10 ppm Zn) R diluted with 0.1 M hydrochloric acid.
Source Zinc hollow-cathode lamp.
Wavelength 213.9 nm.
Atomisation device Air-acetylene flame.
Extractable heavy metals (2.4.8)
Maximum 2 ppm.
Solution S complies with test A. Prepare the reference solution using lead standard solution (2 ppm Pb) R.
Residue on evaporation
Evaporate 50.0 mL of solution S to dryness on a water-bath and dry at 100-105 °C. The residue weighs not more than 2.0 mg for type I closures and not more than 4.0 mg for type II closures.
Volatile sulfides
Place closures, cut if necessary, with a total surface area of 20 ± 2 cm2 in a 100 mL conical flask and add 50 mL of a 20 g/L solution of citric acid monohydrate R. Place a piece of lead acetate paper R over the mouth of the flask and maintain the paper in position by placing over it an inverted weighing bottle. Heat in an autoclave at 121 ± 2 °C for 30 min. Any black stain on the paper is not more intense than that of a standard, treated in the same manner, prepared by mixing 50 mL of a 20 g/L solution of citric acid monohydrate R and 5.0 mL of a freshly prepared 0.0308 g/L solution of sodium sulfide R in water R.
For the tests for penetrability, fragmentation and self-sealing, treat the closures as described for the preparation of solution S and allow to dry.
Penetrability
For closures intended to be pierced by a hypodermic needle, carry out the following test. Fill 10 suitable vials to the nominal volume with water R, fit the closures to be examined and secure with a cap. Using for each closure a new, lubricated, long-bevel1 (bevel angle 12 ± 2°) hypodermic needle with an external diameter of 0.8 mm, pierce the closures with the needle perpendicular to the surface. The force required for piercing, determined with an accuracy of ± 0.25 N, is not greater than 10 N for each closure.
Fragmentation
For closures intended to be pierced by a hypodermic needle, carry out the following test. If the closures are to be used for aqueous preparations, introduce in 12 clean vials a volume of water R corresponding to the nominal volume minus 4 mL, close the vials with the closures to be examined, secure with a cap and allow to stand for 16 h. If the closures are to be used with dry preparations, close 12 clean vials with the closures to be examined. Using a lubricated, long-bevel() (bevel angle 12 ± 2°) hypodermic needle with an external diameter of 0.8 mm fitted to a clean syringe, inject into the vial 1 mL of water R and remove 1 mL of air; carry out this operation 4 times for each closure, piercing the closure each time at a different site. Use a new needle for each closure and check that the needle is not blunted during the test. Pass the liquid in the vials through a filter with a pore size of 0.5 µm. Count the fragments of rubber visible to the naked eye. The total number of fragments does not exceed 5. This limit is based on the assumption that fragments with a diameter equal to or greater than 50 µm are visible to the naked eye; in cases of doubt or dispute, the fragments are examined with a microscope to verify their nature and size.
Self-sealing test
For closures intended to be used with multidose containers, carry out the following test. Fill 10 suitable vials to the nominal volume with water R, fit the closures to be examined and secure with a cap. Using for each closure a new hypodermic needle with an external diameter of 0.8 mm, pierce each closure 10 times, piercing the closure each time at a different site. Immerse the vials upright in a 1 g/L solution of methylene blue R and reduce the external pressure by 27 kPa for 10 min. Restore atmospheric pressure and leave the vials immersed for 30 min. Rinse the outside of the vials. None of the vials contains any trace of coloured solution.