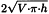Appendix XIX G. Sterile Single-use Plastic Syringes
DEFINITION
Sterile single-use plastic syringes are medical devices intended for immediate use for the administration of injectable preparations. They are supplied sterile and pyrogen-free and are not to be re-sterilised or re-used. They consist of a syringe barrel and a piston which may have an elastomer sealing ring; they may be fitted with a needle which may be non-detachable. Each syringe is presented with individual protection for maintaining sterility.
The barrel of the syringe is sufficiently transparent to permit dosages to be read without difficulty and allow air bubbles and foreign particles to be discerned.
The plastics and elastomer materials of which the barrel and piston are made comply with the appropriate specification or with the requirements of the competent authority. The most commonly used materials are polypropylene and polyethylene. The syringes comply with current standards regarding dimensions and performance.
Silicone oil (3.1.8) may be applied to the internal wall of the barrel to assist in the smooth operation of the syringe but there remains no excess capable of contaminating the contents at the time of use. The inks, glues and adhesives for the marking on the syringe or on the package and, where necessary, the assembly of the syringe and its package, do not migrate across the walls.
TESTS
Solution S
Prepare the solution in a manner that avoids contamination by foreign particles. Using a sufficient number of syringes to produce 50 mL of solution, fill the syringes to their nominal volume with water for injections R and maintain at 37 °C for 24 h. Combine the contents of the syringes in a suitable borosilicate-glass container.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II) and is practically free from foreign solid particles.
Acidity or alkalinity
To 20 mL of solution S add 0.1 mL of bromothymol blue solution R1. Not more than 0.3 mL of 0.01 M sodium hydroxide or 0.01 M hydrochloric acid is required to change the colour of the indicator.
Absorbance (2.2.25)
Maximum 0.40, determined between wavelengths of 220 nm and 360 nm on solution S.
Ethylene oxide
Carrier gas helium for chromatography R.
Flow rate 20 mL/min.
Detection Flame ionisation.
Verify the absence of peaks interfering with the ethylene oxide peak, either by carrying out the test using an unsterilised syringe or using the same chromatographic system with the following modifications:
Ethylene oxide solution Prepare in a fume cupboard. Place 50.0 mL of dimethylacetamide R in a 50 mL vial, stopper, secure the stopper and weigh to the nearest 0.1 mg. Fill a 50 mL polyethylene or polypropylene syringe with gaseous ethylene oxide R, allow the gas to remain in contact with the syringe for about 3 min, empty the syringe and fill again with 50 mL of gaseous ethylene oxide R. Fit a hypodermic needle to the syringe and reduce the volume of gas in the syringe from 50 mL to 25 mL. Inject these 25 mL of ethylene oxide slowly into the vial, shaking gently and avoiding contact between the needle and the liquid. Weigh the vial again: the increase in mass is 45 mg to 60 mg and is used to calculate the exact concentration of the solution (about 1 g/L).
Calibration curve In a series of seven vials of the same type as that used for the test and each containing 150 mL of dimethylacetamide R, place respectively 0 mL, 0.05 mL, 0.10 mL, 0.20 mL, 0.50 mL, 1.00 mL and 2.00 mL of the ethylene oxide solution, i.e. about 0 µg, 50 µg, 100 µg, 200 µg, 500 µg, 1000 µg and 2000 µg of ethylene oxide. Stopper the vials, secure the stoppers and place the vials in an oven at 70 ± 1 °C for 16 h. Inject 1 mL of the hot gas from each vial onto the column and draw a calibration curve from the heights of the peaks and the mass of ethylene oxide in each flask.
Test Weigh the syringe after removing the package. Cut the syringe into pieces of maximum dimension 1 cm and place the pieces in a 250 mL to 500 mL vial containing 150 mL of dimethylacetamide R. Close the vial with a suitable stopper and secure the stopper. Place the vial in an oven at 70 ± 1 °C for 16 h. Remove 1 mL of the hot gas from the vial and inject it onto the column. From the calibration curve and the height of the peak obtained, calculate the mass of ethylene oxide in the vial.
Limit If the label states that ethylene oxide has been used for sterilisation:
Silicone oil
Calculate the internal surface area of a syringe in square centimetres using the following expression:
| V | = | nominal volume of the syringe, in cubic centimetres; |
| h | = | height of the graduation, in centimetres. |
Take a sufficient number of syringes to give an internal surface area of 100 cm2 to 200 cm2. Aspirate into each syringe a volume of methylene chloride R equal to half the nominal volume and make up to the nominal volume with air. Rinse the internal surface corresponding to the nominal volume with the solvent by inverting the syringe ten times in succession with the needle fitting closed by a finger covered by a plastic film inert to methylene chloride. Expel the extracts into a tared dish and repeat the operation. Evaporate the combined extracts to dryness on a water-bath. Dry at 100-105 °C for 1 h. The residue weighs not more than 0.25 mg per square centimetre of internal surface area.
Examine the residue by infrared absorption spectrophotometry (2.2.24). It shows absorption bands typical of silicone oil at 805 cm-1, 1020 cm-1, 1095 cm-1, 1260 cm-1 and 2960 cm-1.
Reducing substances
To 20.0 mL of solution S add 2 mL of sulfuric acid R and 20.0 mL of 0.002 M potassium permanganate. Boil for 3 min. Cool immediately. Add 1 g of potassium iodide R and titrate immediately with 0.01 M sodium thiosulfate using 0.25 mL of starch solution R as indicator. Carry out a blank titration using 20.0 mL of water for injections R. The difference between the titration volumes is not greater than 3.0 mL.
Transparency
Fill a syringe with water R (blank) and fill another with a 1 in 10 dilution of primary opalescent suspension (2.2.1). Use primary opalescent suspension that has been allowed to stand at 20 ± 2 °C for 24 h before use. Compare with the naked eye in diffused light against a dark background. The opalescence of the suspension is detectable when compared with the blank.
Sterility (2.6.1)
Syringes stated to be sterile comply with the test for sterility carried out as follows. Using aseptic technique, open the package, withdraw the syringe, separate the components and place each in a suitable container containing sufficient culture media to cover the part completely. Use both the recommended media (2.6.1).
Syringes stated to be sterile only internally comply with the test for sterility carried out as follows Use 50 mL of inoculation medium for each test syringe. Using aseptic technique, remove the needle protector and submerge the needle in the culture medium. Flush the syringe five times by withdrawing the plunger to its fullest extent.
Pyrogens (2.6.8)
Syringes with a nominal volume equal to or greater than 15 mL comply with the test for pyrogens. Fill a minimum of three syringes to their nominal volume with a pyrogen-free 9 g/L solution of sodium chloride R and maintain at a temperature of 37 °C for 2 h. Combine the solutions aseptically in a pyrogen-free container and carry out the test immediately. Inject per kilogram of the rabbit′s mass 10 mL of the solution.
LABELLING
The label on the package states:
The label on the outer package states: