(Ph. Eur. monograph 1927)
H2O 18.02
Ph Eur
DEFINITION
Water intended for use in the preparation of medicinal products where water of high biological quality is needed, except where Water for injections (0169) is required.
PRODUCTION
Highly purified water is obtained from water that complies with the regulations on water intended for human consumption laid down by the competent authority.
Current production methods include, for example, double-pass reverse osmosis coupled with other suitable techniques such as ultrafiltration and deionisation. Correct operation and maintenance of the system is essential.
In order to ensure the appropriate quality of the water, validated procedures and in-process monitoring of the electrical conductivity and regular microbial monitoring are applied.
Highly purified water is stored in bulk and distributed in conditions designed to prevent growth of micro-organisms and to avoid any other contamination.
Microbiological monitoring
During production and subsequent storage, appropriate measures are taken to ensure that the microbial count is adequately controlled and monitored. Appropriate alert and action levels are set so as to detect adverse trends. Under normal conditions, an appropriate action level is a microbial count of 10 CFU per 100 mL when determined by filtration through a membrane with a nominal pore size not greater than 0.45 µm, using R2A agar, at least 200 mL of highly purified water and incubating at 30-35 °C for not less than 5 days.
R2A agar
Adjust the pH so that after sterilisation it is 7.2 ± 0.2. Sterilise by heating in an autoclave at 121 °C for 15 min.
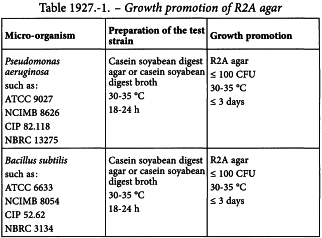
Growth promotion of R2A agar
— Preparation of test strains. Use standardised stable suspensions of test strains or prepare them as stated in Table 1927.-1. Seed lot culture maintenance techniques (seed-lot systems) are used so that the viable micro-organisms used for inoculation are not more than 5 passages removed from the original master seed-lot. Grow each of the bacterial strains separately as described in Table 1927.-1. Use buffered sodium chloride-peptone solution pH 7.0 or phosphate buffer solution pH 7.2 to make test suspensions. Use the suspensions within 2 h, or within 24 h if stored at 2-8 °C. As an alternative to preparing and then diluting a fresh suspension of vegetative cells of Bacillus subtilis, a stable spore suspension is prepared and then an appropriate volume of the spore suspension is used for test inoculation. The stable spore suspension may be maintained at 2-8 °C for a validated period of time.
— Growth promotion. Test each batch of ready-prepared medium and each batch of medium, prepared either from dehydrated medium or from the ingredients described. Inoculate plates of R2A agar separately with a small number (not more than 100 CFU) of the micro-organisms indicated in Table 1927.-1. Incubate under the conditions described in the table. Growth obtained must not differ by a factor greater than 2 from the calculated value for a standardised inoculum. For a freshly prepared inoculum, growth of the micro-organisms must be comparable to that obtained with a previously tested and approved batch of medium.
Total organic carbon (2.2.44)
Maximum 0.5 mg/L.
Determine the conductivity off-line or in-line under the following conditions.
Equipment
Conductivity cell:
— electrodes of a suitable material such as stainless steel;
— cell constant: the cell constant is generally certified by the supplier and is subsequently verified at suitable intervals using a certified reference solution with a
conductivity less than 1500 µS⋅cm
-1 or by comparison with a cell having a certified cell constant; the cell constant is confirmed if the value found is within 2 per cent of the certified value, otherwise re-calibration must be performed.
Conductometer Accuracy of 0.1 µS⋅cm-1 or better at the lowest range.
System calibration (conductivity cell and conductometer):
— against one or more suitable certified reference solutions;
— accuracy: within 3 per cent of the measured
conductivity plus 0.1 µS⋅cm
-1.
Conductometer calibration Calibration is carried out for each range of measurement to be used, after disconnection of the conductivity cell, using certified precision resistors or equivalent devices with an uncertainty not greater than 0.1 per cent of the certified value.
If in-line conductivity cells cannot be dismantled, system calibration may be performed against a calibrated conductivity-measuring instrument with a conductivity cell placed close to the cell to be calibrated in the water flow.
Temperature measurement Tolerance ± 2 °C.
PROCEDURE
Stage 1
1. Measure the
conductivity without temperature compensation, recording simultaneously the temperature. Temperature-compensated measurement may be performed after suitable validation.
2. Using Table 1927.-2, find the closest temperature value that is not greater than the measured temperature. The corresponding
conductivity value is the limit at that temperature.
3. If the measured
conductivity is not greater than the value in Table 1927.-2, the water to be examined meets the requirements of the test for
conductivity. If the
conductivity is higher than the value in Table 1927.-2, proceed with stage 2.
Stage 2
4. Transfer a sufficient amount of the water to be examined (100 mL or more) to a suitable container, and stir the test sample. Adjust the temperature, if necessary, and while maintaining it at 25 ± 1 °C, begin vigorously agitating the test sample while periodically observing the
conductivity. When the change in
conductivity (due to uptake of atmospheric carbon dioxide) is less than 0.1 µS⋅cm
-1 per 5 min, note the
conductivity.
5. If the
conductivity is not greater than 2.1 µS⋅cm
-1, the water to be examined meets the requirements of the test for
conductivity. If the
conductivity is greater than 2.1 µS⋅cm
-1, proceed with stage 3.
Stage 3
6. Perform this test within approximately 5 min of the
conductivity determination in step 5 under stage 2, while maintaining the sample temperature at 25 ± 1 °C. Add a recently prepared saturated solution of
potassium chloride R to the test sample (0.3 mL per 100 mL of the test sample), and determine the pH (
2.2.3) to the nearest 0.1 pH unit.
7. Using Table 1927.-3, determine the
conductivity limit at the measured pH value in step 6. If the measured
conductivity in step 4 under stage 2 is not greater than the
conductivity requirements for the pH determined, the water to be examined meets the requirements of the test for
conductivity. If either the measured
conductivity is greater than this value or the pH is outside the range of 5.0-7.0, the water to be examined does not meet the requirements of the test for
conductivity.
CHARACTERS
Appearance
Clear and colourless liquid.
TESTS
Nitrates
Maximum 0.2 ppm.
Place 5 mL in a test-tube immersed in iced water, add 0.4 mL of a 100 g/L solution of potassium chloride R, 0.1 mL of diphenylamine solution R and, dropwise with shaking, 5 mL of nitrogen-free sulfuric acid R. Transfer the tube to a water-bath at 50 °C. After 15 min, any blue colour in the solution is not more intense than that in a reference solution prepared at the same time in the same manner using a mixture of 4.5 mL of nitrate-free water R and 0.5 mL of nitrate standard solution (2 ppm NO3) R.
Maximum 10 ppb, if intended for use in the manufacture of dialysis solutions.
Prescribed solution To 400 mL of the water to be examined add 10 mL of acetate buffer solution pH 6.0 R and 100 mL of distilled water R.
Reference solution Mix 2 mL of aluminium standard solution (2 ppm Al) R, 10 mL of acetate buffer solution pH 6.0 R and 98 mL of distilled water R.
Blank solution Mix 10 mL of acetate buffer solution pH 6.0 R and 100 mL of distilled water R.
Less than 0.25 IU/mL.
LABELLING
The label states, where applicable, that the substance is suitable for use in the manufacture of dialysis solutions.
Ph Eur