SC VII C. Monographs on Herbal Drug Extracts (Information Chapter)
Basis for elaboration of monographs on herbal drug extract
European Pharmacopoeia monographs on herbal drug extracts are elaborated on the basis of extracts present in medicinal products that have been authorised and/or registered by the competent authorities of Parties to the Convention on the Elaboration of a European Pharmacopoeia. However, these monographs do not necessarily cover all extracts which may be available on the market.
European Pharmacopoeia monographs on herbal drug extracts are elaborated by groups of experts and working parties in collaboration with national pharmacopoeia authorities, the competent authorities for marketing authorisation, Official Medicines Control Laboratories (OMCLs) and the EDQM laboratory; they are also assisted by the producers of herbal drug extracts and/or the pharmaceutical manufacturers that use these extracts.
During the elaboration of an extract monograph, the group of experts works on the basis of a number of extracts from the specified herbal drug that are incorporated into authorised and/or registered medicinal products originating from different sources. These extracts may have been produced using different extraction solvents and/or extraction processes and may include different types and contents of excipients (added for technological purposes during production of the extract).
Where the results from the analysis of the extracts indicate that all of the extracts are in compliance with all of the quality parameters, the monograph is intended to apply to all types of production of that specific herbal extract (for example, Devil’s claw dry extract (1871), where differences in the extraction solvent used, as stated in the Production section of the monograph, and any differences between the extracts in terms of their production process have no significant effect on the quality parameters).
Where there is a definable difference in the quality parameters between the extracts due to one or more aspects of the production process, the monograph is presented as a family monograph in which there will be a qualitative or quantitative difference applicable to one or more analytical parameters (for example, Boldo leaf dry extract (1816), where differences in the extraction solvents used, as stated in the Production section of the monograph, necessitates defining a lower minimum content of assayed constituents in the aqueous extract in comparison with the minimum content in the hydroalcoholic extract; all other quality parameters are identical).
Where there is a significant difference in the quality parameters between the extracts due to one or more aspects of the production process, more than one extract monograph is elaborated (for example, Valerian dry aqueous extract (2400) and Valerian dry hydroalcoholic extract (1898), where differences in the extraction solvents used and the processing method, as stated in the Production section of the monographs, necessitates defining different minimum values for the assayed constituents and different chromatographic profiles for the aqueous and the hydroalcoholic extracts).
Types of extract
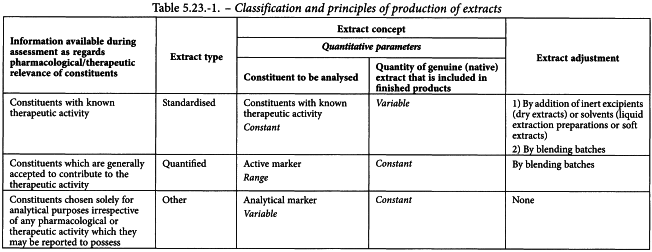
The general monograph Herbal drug extracts (0765) distinguishes different types of extracts. This classification is based on the principles applied by European competent authorities during the assessment of extracts in applications for marketing authorisation/registration of medicinal products. These principles are summarised in Table 5.23.-1.
This classification of extracts implies that, for each of these types of extract, distinct principles of production and of defining or adjusting the content of assayed constituents are required by the general monograph Herbal drug extracts (0765).
Genuine (native) extract The concept of the genuine (native) drug extract ratio (DERgenuine) (see the Glossary in the monograph Herbal drug extracts (0765)) was originally devised for application to dry extracts where, after extraction of the herbal drug, all solvent is removed leaving only the extracted dry matter from the herbal drug, that is, the genuine (native) extract. However, this extracted dry matter often requires additives (inert excipients as processing aids) to produce a technologically suitable extract. Such extracts made by different manufacturers from a given herbal drug may have been produced using different solvents and processing methods and contain different amounts of excipients relative to the quantity of extracted dry matter. In order to permit comparison of such extracts, the declaration of the drug extract ratio (DER) on the basis of the genuine (native) extract was introduced. Thus, for those dry extracts which can be produced without excipients, the DER and DERgenuine are identical but, where excipients are required, the DER and DERgenuine are different. It was then thought necessary to apply this concept to soft extracts and liquid extraction preparations where solvent and, in some cases, other substances are present as an integral part of the extract. For these extracts, the concept of basing the DERgenuine on extracted dry matter was abandoned and the extract in its entirety (including solvents, processing aids, etc.) is considered to be the genuine (native) extract. Therefore, for these extracts the DER and DERgenuine are identical.
Constituents for assay
For the purposes of quality control, monographs of the European Pharmacopoeia generally include an assay. The choice of the constituent(s) to be assayed is linked, wherever possible, to the regulatory process and is based upon the following criteria:
Use of analytical markers in ‘other’ extracts
The assay method described in an individual monograph:
The minimum content of the analytical marker when using the assay method:
Since the monograph may encompass a broad range of extracts, the minimum value given for the analytical marker is not regarded as a stand-alone quality criterion (as compared to other minimum values given in the Pharmacopoeia). However, it gives an indication of the minimum values that can be expected when producing an extract from a herbal drug compliant with its monograph.
Based on the information given above, manufacturers may be requested by competent authorities to complement this information with a minimum value for their own extract depending on their individual manufacturing process and excipients added.
NOTE: information on the characteristics of the extracts (for example, strength of extraction solvent, percentage of genuine extract, etc.) analysed during the elaboration of an individual extract monograph will be made available to users of the European Pharmacopoeia in the Knowledge database on the EDQM website.