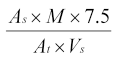Erythropoietin Injection
Action and use
Erythropoietin analogue.
Definition
Erythropoietin Injection is a sterile solution of Erythropoietin Concentrated Solution in a suitable liquid. It is either supplied as a ready-to-use solution or it is prepared by dissolving Erythropoietin for Injection in the liquid stated on the label.
Production
Where the product contains human serum albumin, it does not comply with the test for Dimers and related substances of higher molecular weight; however the manufacturing process is validated to show that aggregation does not occur.
Potency
The estimated potency is not less than 80% and not more than 125% of the stated potency.
Characteristics
A clear, colourless solution virtually free from particles.
Identification
Tests
Acidity or alkalinity
pH, 6.6 to 7.4, Appendix V L.
Dimers and related substances of higher molecular weight
Use method A or method B.
0.115% w/v of anhydrous disodium hydrogen orthophosphate, 0.02% w/v of potassium dihydrogen orthophosphate and 2.34% w/v of sodium chloride in water; if necessary, adjust to pH 7.4.
The test is not valid unless:
in the chromatogram obtained with solution (3) aggregates are present, the resolution factor between the aggregate and monomer peaks is at least 0.8 and the relative standard deviation is less than 10%;
in the chromatogram obtained with solution (2) the area of the principal peak is 1.5 to 2.5% of the area of the principal peak in the chromatogram obtained with the solution (1).
In the chromatogram obtained with solution (1) the total area of any peaks eluting before the principal peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (2.0%).
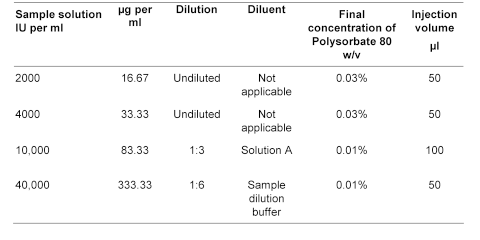
The sample dilution buffer contains 2 volumes of solution A and 1 volume of solution B.
25% v/v of propanol-2-ol in Solution A.
The test is not valid unless:
solution B shows no interfering peaks;
in the chromatogram obtained with solution (1), aggregates are present and the resolution factor between the aggregate and monomer peaks is at least 0.8.
In the chromatogram with solution (2) the total area of any peaks eluting before the principal peak is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (2.0%).
Bacterial endotoxins
Carry out the test for bacterial endotoxins, Appendix XIV C. The endotoxin limit concentration is less than 20 IU in a volume containing 10,000 IU of Erythropoietin.
Assay
Carry out the Assay using method A or method B.
The activity of the preparation is compared with that of erythropoietin EPBRP and expressed in International Units (IU).
The confidence limits of the estimated potency (P = 0.95) are not less than 64% and not more than 156% of the stated potency.
A. In polycythaemic mice
The activity of the preparation is estimated by examining, under given conditions, its effect in stimulating the incorporation of 59Fe into circulating red blood cells of mice made polycythaemic by exposure to reduced atmospheric pressure.
The following schedule, using treatment in a hypobaric chamber, has been found to be suitable.
Induce polycythaemia in female mice of the same strain, weighing 16 to 18 g. Place the mice in a hypoxic chamber and reduce the pressure to 0.6 atmospheres. After 3 days at 0.6 atmospheres, further reduce the pressure to 0.4 to 0.5 atmospheres and maintain the animals at this pressure for a further 11 days (the partial vacuum is interrupted daily for a maximum of 1 hour at about 11:00 a.m., in order to clean the cages and feed the animals). At the end of the specified period, return the mice to normal atmospheric conditions. Randomly distribute the mice into cages, each containing 6 animals, and mark them.
Test solution (1) Dilute the substance to be examined in phosphate-albumin buffered saline pH 7.2 R1 to obtain a concentration of 0.2 IU per mL.
Test solution (2) Mix equal volumes of test solution (1) and phosphate-albumin buffered saline pH 7.2 R1.
Test solution (3) Mix equal volumes of test solution (2) and phosphate-albumin buffered saline pH 7.2 R1.
Reference solution (1) Dissolve erythropoietin EPBRP in phosphate-albumin buffered saline pH 7.2 R1 to obtain a concentration of 0.2 IU per mL.
Reference solution (2) Mix equal volumes of reference solution (1) and phosphate-albumin buffered saline pH 7.2 R1.
Reference solution (3) Mix equal volumes of reference solution (2) and phosphate-albumin buffered saline pH 7.2 R1.
Radiolabelled ferric [59Fe] chloride solution, concentrated Use a commercially available solution of [59Fe] ferric chloride (approximate specific activity: 100 to 1000 MBq per mg of Fe).
Radiolabelled [59Fe] ferric chloride solution Dilute the concentrated radiolabelled [59Fe] ferric chloride solution in sodium citrate buffer solution pH 7.8 to obtain a solution with an activity of 3.7 × 104 Bq per mL.
The concentrations of the test solutions and reference solutions may need to be modified, based on the response range of the animals used.
Three days after returning the animals to atmospheric pressure, inject each animal subcutaneously with 0.2 mL of one of the solutions. The 6 animals in each cage must each receive one of the 6 different treatments (3 test solutions and 3 reference solutions); the order of injection must be separately randomised for each cage. A minimum of 8 cages is recommended. Two days after injection of the test or reference solution, inject each animal intraperitoneally with 0.2 mL of radiolabelled [59Fe] ferric chloride solution. The order of the injections must be the same as that of the erythropoietin injections, and the time interval between administration of the erythropoietin and the radiolabelled ferric chloride solution must be the same for each animal. After a further 48 hours, anaesthetise each animal by injection of a suitable anaesthetic, record body weights and withdraw blood samples (0.65 mL) into haematocrit capillaries from the bifurcation of the aorta. After determining the packed cell volume for each sample, measure the radioactivity.
Calculate the response (percentage of 59Fe in total circulating blood) for each mouse using the expression:
As | = | radioactivity in the sample, |
At | = | total radioactivity injected, |
7.5 | = | total blood volume as per cent body weight, |
M | = | body weight, in grams, |
Vs | = | sample volume. |
Calculate the potency by the usual statistical methods for a parallel line assay. Eliminate from the calculation any animal where the packed cell volume is less than 54%, or where the body weight is more than 24 g.
B. In normocythaemic mice
The Assay is based on the measurement of stimulation of reticulocyte production in normocythaemic mice.
The Assay may be carried out using the following procedure:
Test solution (1) Dilute the preparation being examined in phosphate-albumin buffered saline pH 7.2 R1 to obtain a concentration of 80 IU per mL.
Test solution (2) Mix equal volumes of test solution (1) and phosphate-albumin buffered saline pH 7.2 R1.
Test solution (3) Mix equal volumes of test solution (2) and phosphate-albumin buffered saline pH 7.2 R1.
Reference solution (1) Dissolve erythropoietin EPBRP in phosphate-albumin buffered saline pH 7.2 R1 to obtain a concentration of 80 IU per mL.
Reference solution (2) Mix equal volumes of reference solution (1) and phosphate-albumin buffered saline pH 7.2 R1.
Reference solution (3) Mix equal volumes of reference solution (2) and phosphate-albumin buffered saline pH 7.2 R1.
The exact concentrations of the test solutions and reference solutions may need to be modified, based on the response range of the animals used.
At the beginning of the assay procedure, randomly distribute mice of a suitable age and strain (8-week old B6D2F1 mice are suitable) into 6 cages. A minimum of 8 mice per cage is recommended. Inject each animal subcutaneously with 0.5 mL of the appropriate treatment (one solution per cage) and put the animal in a new cage. Combine the mice in such a way that each cage housing the treated mice contains one mouse out of the 6 different treatments (3 test solutions and 3 reference solutions, 6 mice per cage). 4 days after the injections, collect blood samples from the animals and determine the number of reticulocytes using a suitable procedure.
The following method may be employed:
The volume of blood, dilution procedure and fluorescent reagent may need to be modified to ensure maximum development and stability of fluorescence.
Colorant solution, concentrated Use a solution of thiazole orange suitable for the determination of reticulocytes. Prepare at a concentration twice that necessary for the analysis.
Proceed with the following dilution steps. Dilute whole blood 500-fold in the buffer used to prepare the colorant solution. Dilute this solution 2-fold in the concentrated colorant solution. After staining for 3 to 10 minutes, determine the reticulocyte count microfluorometrically in a flow cytometer. The percentage of reticulocytes is determined using a biparametric histogram: number of cells/red fluorescence (620 nm).
Calculate the potency by the usual statistical methods for a parallel line assay.
Storage
Erythropoietin Injection should be protected from light.
Labelling
The label states the number of IU (Units) in a suitable dose-volume.
ERYTHROPOIETIN FOR INJECTION
Definition
Erythropoietin for Injection is a freeze-dried, sterile preparation prepared from Erythropoietin Concentrated Solution. It is supplied in a sealed container.
The contents of the sealed container comply with the requirements for Powders for Injections or Infusions stated under Parenteral Preparations and with the following requirements.
Identification
Tests
Dimers and related substances of higher molecular weight
Comply with the test described for Erythropoietin Injection using the following solutions.
Water
Not more than 4.0% w/w, Appendix IX C, Method 1.
Bacterial endotoxins
Carry out the test for bacterial endotoxins, Appendix XIV C. The endotoxin limit concentration is less than 20 IU in a volume containing 10,000 IU of Erythropoietin.
Assay
Carry out the Assay as described for Erythropoietin Injection using the following solutions:
A. In polycythaemic mice
Test solution (1) 0.2 IU per mL in phosphate-albumin buffered saline pH 7.2 R1.
B. In normocythaemic mice
Test solution (1) 80 IU per mL in phosphate-albumin buffered saline pH 7.2 R1.
Storage
Erythropoietin Injection prepared by dissolving the contents of a sealed container in the liquid stated on the label should be used immediately after preparation but, in any case, within the period recommended by the manufacturer when prepared and stored strictly in accordance with the manufacturer′s instructions.
Labelling
The label of the sealed container states the number of IU (Units) contained in it.