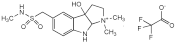
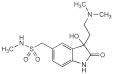
Sumatriptan Injection
Action and use
Serotonin 5HT1 receptor agonist; treatment of migraine.
Definition
Sumatriptan Injection is a sterile isotonic solution of Sumatriptan Succinate in Water for Injections.
Content of sumatriptan, C14H21N3O2S
95.0 to 105.0% of the stated amount.
Characteristics
A clear, colourless to pale yellow solution.
Identification
To a volume of the injection containing the equivalent of 29 mg of sumatriptan add 1 mL of saturated sodium chloride solution and 1 mL of saturated sodium carbonate solution. Shake vigorously for 30 seconds, add two 2-mL quantities of propan-2-ol, shake, allow to separate (this may take up to 24 hours) and discard the aqueous layer. Evaporate under a stream of nitrogen and dry at 100°. The infrared absorption spectrum is concordant with the reference spectrum of sumatriptan (RS 414).
Tests
Acidity
pH, 4.2 to 5.3, Appendix V L.
Impurities A and H
Carry out the method for liquid chromatography, Appendix III D. Prepare the solutions in a mixture containing 3 volumes of 0.025M sodium dihydrogen orthophosphate, the pH of which has been adjusted to 6.5, and 1 volume of acetonitrile.
10 volumes of 10m ammonium acetate and 90 volumes of methanol.
The test is not valid unless in the chromatogram obtained with solution (3):
the chromatogram resembles that supplied with sumatriptan for system suitability EPCRS;
the resolution between impurity A and sumatriptan is at least 1.5.
Identify any peak in the chromatogram obtained with solution (1) due to impurity A using the chromatogram obtained with solution (3). Multiply the area of any peak corresponding to impurity A by a correction factor of 0.6.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity A is not greater than 1.5 times the area of the principal peak in the chromatogram obtained with solution (2) (1.5%);
the area of any peak corresponding to impurity H is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1.0%).
Related substances
Carry out the method for liquid chromatography, Appendix III D. Prepare the solutions in a mixture containing 3 volumes of 0.025M sodium dihydrogen orthophosphate, the pH of which has been adjusted to 6.5, and 1 volume acetonitrile.
25 volumes of acetonitrile and 75 volumes of a solution containing 0.97 g of dibutylamine, 0.735 g of orthophosphoric acid and 2.93 g of sodium dihydrogen orthophosphate in 750 mL of water, adjusted to pH 7.5 with 10m sodium hydroxide and diluted to 1000 mL with water.
The test is not valid unless, in the chromatagram obtained solution (3):
the chromatogram resembles that supplied with sumatriptan impurity mixture EPCRS;
the resolution between impurity C and sumatriptan is at least 1.5.
Identify any peaks in the chromatogram obtained with solution (1) due to impurity 1 and impurity 2 using the chromatogram obtained with solution (4) and the reference chromatogram supplied with sumatriptan impurity standard BPCRS. Multiply the area of any peak corresponding to impurity 1 by a correction factor of 0.2 and multiply the area of any peak corresponding to impurity 2 by a correction factor of 0.3.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity 1 is not greater than 1.5 times the area of the principal peak in the chromatogram obtained with solution (2) (1.5%);
the area of any peak corresponding to impurity 2 is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1.0%);
the area of any other secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1.0%).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (5) (0.1%).
The total impurity content in the test for Impurities A and H and the test for Related substances is not greater than 4.0%.
Bacterial endotoxins
Carry out the test for bacterial endotoxins, Appendix XIV C. Dilute the injection, if necessary, with water BET to give a solution containing the equivalent of 3.6 mg of sumatriptan per mL (solution A). The endotoxin limit concentration is less than 350.0 IU of endotoxin per mL.
Assay
Carry out the method for liquid chromatography, Appendix III D. Prepare the solutions in a mixture containing 3 volumes of 0.025m sodium dihydrogen orthophosphate, the pH of which has been adjusted to 6.5, and 1 volume of acetonitrile.
The chromatographic conditions described under Related substances may be used.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between impurity C and sumatriptan is at least 1.5.
Calculate the content of C14H21N3O2S in the injection using the declared content of C14H21N3O2S,C4H6O4 in sumatriptan succinate BPCRS. 1 mg of C14H21N3O2S is equivalent to 1.400 mg of C14H21N3O2S,C4H6O4.
Storage
Sumatriptan Injection should be protected from light.
Labelling
The quantity of the active ingredient is stated in terms of the equivalent amount of sumatriptan.
Impurities
The impurities limited by the requirements of this monograph include those listed under Sumatriptan Succinate and the following: