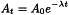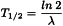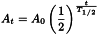Radiopharmaceutical Preparations
Ph Eur
DEFINITIONS
Radiopharmaceutical preparations or radiopharmaceuticals are medicinal products which, when ready for use, contain 1 or more radionuclides (radioactive isotopes) included for a medicinal purpose.
For the purpose of this general monograph, radiopharmaceutical preparations also cover:
Radionuclide precursors may be supplied as solutions for radiolabelling.
A nuclide is a species of atom characterised by the number of protons and neutrons in its nucleus (and hence by its atomic number Z and mass number A) and also by its nuclear energy state. Isotopes of an element are nuclides with the same atomic number but different mass numbers. Nuclides containing an unstable arrangement of protons and neutrons will transform spontaneously to either a stable or another unstable combination of protons and neutrons with a constant statistical probability. Such nuclides are said to be radioactive and are called radionuclides. The initial unstable nuclide is referred to as the parent radionuclide and the resulting nuclide as the daughter nuclide.
Decay or transformation of radionuclides may involve the emission of charged particles, electron capture (EC) or isomeric transition (IT). The charged particles emitted from nuclei may be alpha particles (nuclei of 4He) or beta particles (negatively charged, generally called electrons, or positively charged, generally called positrons). Alpha decay usually concerns heavy nuclei (Z > 82). Radionuclides with a deficit of protons usually decay by emitting electrons. Radionuclides with a deficit of neutrons usually decay by electron capture or by emitting positrons. In the latter case, radionuclides are called positron emitters. Positrons are annihilated after interaction with electrons in the surrounding matter. The annihilation results in the emission of 2 gamma photons, each with energy of 0.511 MeV, generally emitted at 180° to each other (annihilation radiation). All decay modes may be accompanied by an emission of gamma rays. The emission of gamma rays may be partly or completely replaced by the ejection of electrons, known as internal conversion electrons. This phenomenon, like the process of electron capture, causes a secondary emission of X-rays (due to a reorganisation of the electrons in the atom). This secondary emission may itself be partly replaced by the ejection of electrons, known as Auger electrons.
Radioactivity
Generally the term ‘radioactivity′ is used both to describe the phenomenon of radioactive decay and to express the physical quantity of this phenomenon.
The radioactivity of a preparation is the number of nuclear disintegrations or transformations per unit time.
In the International System (SI), radioactivity is expressed in becquerel (Bq), which is 1 nuclear transformation per second. Absolute radioactivity measurements require a specialised laboratory but identification of radioactivity and quantitative measurement of radioactivity can be carried out relatively by comparing the measured samples with standardised preparations provided by laboratories recognised by the competent authority or by using a calibrated instrument.
Radioactive decay
Any radionuclide decays at an exponential rate with its characteristic decay constant. The curve of exponential decay (decay curve) is described by the following expression:
At | = | the radioactivity at time t; |
A0 | = | the radioactivity at time t = 0; |
λ | = | the decay constant, characteristic of each radionuclide; |
e | = | the base of natural logarithms. |
The half-life (T1/2) is the time in which a given radioactivity (amount) of a radionuclide decays to half its initial value.
It is related to the decay constant (λ) by the following equation:
The equation of exponential decay can thus be expressed also in the following way, useful for the fast estimation of the radioactivity left after elapsing time t:
The penetrating power of each radiation varies considerably according to its nature and its energy. Alpha particles are completely absorbed in a thickness of a few micrometres to some tens of micrometres of matter. Beta particles are completely absorbed in a thickness of several millimetres to several centimetres of matter. Gamma rays are not completely absorbed but only attenuated and a tenfold reduction may require, for example, several centimetres of lead. The denser the absorbent, the shorter the range of alpha and beta particles and the greater the attenuation of gamma rays.
Each radionuclide is characterised by an invariable half-life, expressed in units of time and by the nature and energy of its radiation or radiations. The energy is expressed in electronvolts (eV), kilo-electronvolts (keV) or mega-electronvolts (MeV).
Radionuclidic purity
The ratio, expressed as a percentage, of the radioactivity of the radionuclide concerned to the total radioactivity of the radiopharmaceutical preparation. The relevant potential radionuclidic impurities are listed with their limits in the individual monographs.
Radiochemical purity
The ratio, expressed as a percentage, of the radioactivity of the radionuclide concerned which is present in the radiopharmaceutical preparation in the stated chemical form, to the total radioactivity of that radionuclide present in the radiopharmaceutical preparation. The relevant potential radiochemical impurities are listed with their limits in the individual monographs.
Chemical purity
In monographs on radiopharmaceutical preparations, chemical purity is controlled by specifying limits for chemical impurities.
Isotopic carrier
A stable isotope of the element concerned either present in or added to the radioactive preparation in the same chemical form as that in which the radionuclide is present.
Carrier-free preparation
A preparation free from stable isotopes of the same element as the radionuclide concerned present in the preparation in the stated chemical form or at the position of the radionuclide in the molecule concerned.
No-carrier-added preparation
A preparation to which no stable isotopes of the same element as the radionuclide concerned are intentionally added in the stated chemical form or at the position of the radionuclide in the molecule concerned.
Specific radioactivity
The radioactivity of a radionuclide per unit mass of the element or of the chemical form concerned, e.g. becquerel per gram or becquerel per mole.
Radioactivity concentration
The radioactivity of a radionuclide per unit volume or unit mass of the preparation. For radiopharmaceutical solutions, it is expressed as radioactivity per unit volume of the preparation.
Total radioactivity
The radioactivity of the radionuclide, expressed per unit (vial, capsule, ampoule, generator, etc).
Period of validity
The time during which specifications described in the monograph must be fulfilled.
PRODUCTION
A radiopharmaceutical preparation contains its radionuclide:
Radionuclides can be produced in the following ways:
The probability of nuclear reaction occurrence depends on the nature and energy of the incident particles (protons, neutrons, deuterons etc.) and on the nature of the nucleus that is irradiated by them. The rate of production (yield) of a given radionuclide resulting from the irradiation depends in addition on the isotopic composition of the target material and its chemical purity, and in the case of neutrons on their flux, and in the case of charged particles on beam current.
In addition to the desired nuclear reaction, simultaneous transformations usually occur. Probability of their occurrence is given by the same factors as mentioned in the previous paragraph. Such simultaneous transformations may give rise to radionuclidic impurities.
The nuclear reaction (transformation) can be written in the form: target nucleus (incident particle, emitted particle) produced nucleus.
neutron irradiation
Irradiation of stable radionuclides in nuclear reactors usually results in proton-deficient nuclei, i.e. electron emitters that are formed in (n,γ) reactions (so-called radiative capture). The product is isotopic with the target nucleus and it may thus contain a considerable amount of carrier.
A number of nuclides with high atomic number are fissionable by neutrons. Nuclear fission, denoted as (n, f) reaction, results in a large number of radionuclides of various masses and half-lives. The most frequently used fission is that of 235U. Iodine-131, molybdenum-99 and xenon-133 can be produced by irradiation of 235U in nuclear reactors and by their separation from more than 200 radionuclides formed in that process.
charged particle irradiation
Irradiation of stable radionuclides with charged particles usually results in neutron-deficient nuclei that decay either by electron capture or by positron emission. They are formed in particular in (p, xn) reactions (where x is the number of emitted neutrons). The product is not isotopic with the target nucleus and its specific radioactivity might be close to that of a carrier-free preparation.
Radionuclide generators
Radionuclide generator systems use a parent radionuclide which decays to a daughter radionuclide with a shorter half-life.
By separating the daughter radionuclide from the parent radionuclide by a chemical or physical process, it is possible to use the daughter radionuclide at a considerable distance from the production site of the generator despite its short half-life.
Target materials
The isotopic composition and purity of the target material together with other factors such as the nature and energy of incident particles will determine the relative percentages of the principal radionuclide and radionuclidic impurities produced by irradiation. The use of isotopically enriched target material in which the abundance of the required target nuclide has been artificially increased, can improve the production yield and the purity of the desired radionuclide.
The chemical form, the purity and the physical state of the target material and the chemical additives, as well as the irradiation conditions and the direct physical and chemical environment, determine the chemical state and chemical purity of the radionuclides that are produced. In the production of radionuclides, and particularly of radionuclides with a short half-life, it may not be possible to determine any of these quality criteria before further processing and manufacture of radiopharmaceutical preparations. Therefore the quality of each batch of target material is assessed before its use in routine radionuclide production and manufacture of radiopharmaceutical preparations.
The target material is contained in a holder in gaseous, liquid or solid state, in order to be irradiated by a beam of particles. For neutron irradiation, the target material is commonly contained in quartz ampoules or high-purity aluminium or titanium containers. It is necessary to ascertain that no interaction can occur between the container and its contents under the irradiation conditions.
For charged particle irradiation, the holder for target material is constructed of an appropriate metal, possibly with inlet and outlet ports, a surrounding cooling system and usually a thin metal foil target window.
To evaluate all effects on the efficiency of the production of the radionuclide in terms of quality and quantity, the production procedure must clearly describe and take into consideration: the target material, the construction of the holder for target material, method of irradiation and separation of the desired radionuclide.
CHARACTERS
The general chapter 5.7 Table of physical characteristics of radionuclides mentioned in the European Pharmacopoeia summarises the most commonly accepted physical characteristics of radionuclides used in preparations that are the subject of monographs in the European Pharmacopoeia. In addition, the Table states the physical characteristics of the main potential radionuclidic impurities of the radionuclides mentioned in the monographs.
The term ‘transition probability’ means the probability of the transformation of a nucleus in a given energy state, via the transition concerned. Instead of ‘probability’ the term ‘abundance’ is also used.
The term ‘emission probability’ means the probability that an atom of a radionuclide gives rise to the emission of the particles or radiation concerned.
Irrespective of which meaning is intended, probability is usually stated as a percentage.
IDENTIFICATION
A radionuclide is generally identified by its half-life or by the nature and energy of its radiation or radiations or by both, as prescribed in the monograph.
Approximate half-life
The half-life as determined over a relatively short time period to allow release for use of radiopharmaceutical preparations.
The calculated approximate half-life is within the range of the values stated in the individual monograph.
Determination of the nature and energy of the radiation
The nature and energy of the radiation emitted are determined using spectrometry. The nature and energy of the radiation of positron emitters is usually not determined; their identification is performed by determination of their half-life and gamma-ray spectrum.
TESTS
It is sometimes difficult to carry out some of the following tests before releasing the batch for use when the half-life of the radionuclide in the preparation is short. The individual monograph indicates the tests that need not be completed before release for use. These tests then constitute a control of the quality of production.
Non-radioactive substances and related substances
This section prescribes the determination of non-radioactive substances and related substances that can be present.
Residual solvents
Residual solvents are limited according to general chapter 5.4. Residual solvents, using the methods given in general chapter 2.4.24. Identification and control of residual solvents or another suitable method.
RADIONUCLIDIC PURITY
Radionuclidic impurities may arise during the production and decay of a radionuclide. Potential radionuclidic impurities may be mentioned in the monographs and their characteristics are described in general chapter 5.7. Table of physical characteristics of radionuclides mentioned in the European Pharmacopoeia.
In most cases, to establish the radionuclidic purity of a radiopharmaceutical preparation, the identity of every radionuclide present and its radioactivity must be known. Generally, the most useful method for examination of the radionuclidic purity of gamma- and X-ray emitting radionuclides is gamma-ray spectrometry. The use of sodium iodide detectors may cause a problem: the peaks due to gamma-ray emitting impurities may be concealed in the spectrum of the principal radionuclide or left unresolved from peaks of other radionuclidic impurities in the preparation. Alpha- and beta-particle emitting impurities that do not emit gamma- or X-rays cannot be detected in this way. For alpha- and beta-emitters other methods must be employed.
The individual monographs prescribe the radionuclidic purity required and may set limits for specific radionuclidic impurities (for example, molybdenum-99 in technetium-99m). While these requirements are necessary, they are not in themselves sufficient to ensure that the radionuclidic purity of a preparation is sufficient for its clinical use. The manufacturer must examine the product in detail and especially must examine preparations of radionuclides with a short half-life for impurities with a long half-life after a suitable period of decay. In this way, information on the suitability of the manufacturing processes and the adequacy of the testing procedures is obtained. In cases where 2 or more positron-emitting radionuclides need to be identified and/or differentiated, for example the presence of 18F-impurities in 13N-preparations, half-life determinations need to be carried out in addition to gamma-ray spectrometry.
Due to differences in the half-lives of the different radionuclides present in a radiopharmaceutical preparation, the radionuclidic purity changes with time.
RADIOCHEMICAL PURITY
Radiochemical impurities may originate from:
The determination of radiochemical purity requires separation of the different chemical substances containing the radionuclide and determination of the percentage of radioactivity of the radionuclide concerned associated with the stated chemical form. The radiochemical purity section of an individual monograph may include limits for specified radiochemical impurities, including isomers.
In principle, any method of analytical separation may be used in the determination of radiochemical purity. For example, the monographs for radiopharmaceutical preparations may include paper chromatography (2.2.26), thin-layer chromatography (2.2.27), electrophoresis (2.2.31), size-exclusion chromatography (2.2.30), gas chromatography (2.2.28) and liquid chromatography (2.2.29). The technical description of these analytical methods is set out in the monographs. Moreover, certain precautions special to radiopharmaceuticals must also be considered, such as radiation protection, measurement geometry, detector linearity, use of carriers, dilution of the preparation.
Specific radioactivity
Specific radioactivity is usually calculated taking into account the radioactivity concentration and the concentration of the chemical substance being studied, after verification that the radioactivity is attributable only to the radionuclide (radionuclidic purity) and the chemical species (radiochemical purity) concerned.
Specific radioactivity changes with time. The statement of the specific radioactivity therefore includes reference to a date and, if necessary, time.
Physiological distribution
Tests involving animals should be avoided wherever possible. Where the tests for identity and for radiochemical purity are not adequate to completely define and control the radiochemical species in a radiopharmaceutical preparation, a physiological distribution test may be required. The distribution pattern of radioactivity observed in specified organs, tissues or other body compartments of an appropriate animal species can be a reliable indication of the suitability for the intended purpose.
Alternatively, a physiological distribution test can serve to establish the biological equivalence of the preparation under test with similar preparations known to be clinically effective.
The individual monograph prescribes the details concerning the conduct of the test and the physiological distribution requirements that must be met.
In general, the test is performed as follows.
Each of 3 animals is injected intravenously with the preparation. In some cases, dilution immediately before injection may be necessary.
Immediately after injection each animal is placed in a separate cage for collection of excreta and prevention of contamination of the body surface of the animal. At the specified time after injection, the animals are euthanised by an appropriate method and dissected. Selected organs and tissues are assayed for their radioactivity. The physiological distribution is then calculated and expressed in terms of the percentage of the administered radioactivity that is found in each of the selected organs or tissues, taking into account corrections for radioactive decay. For some radiopharmaceutical preparations it is necessary to determine the ratio of the radioactivity in weighed samples of selected tissues (radioactivity/mass).
A preparation meets the requirements of the test if the distribution of radioactivity in at least 2 of the 3 animals complies with all the specified criteria.
Disregard the results from any animal showing evidence of extravasation of the injection (observed at the time of injection or revealed by subsequent assay of tissue radioactivity). In that case the test may be repeated.
Sterility
Radiopharmaceutical preparations for parenteral administration comply with the test for sterility. They must be prepared using precautions designed to exclude microbial contamination and to ensure sterility. The test for sterility is carried out as described in the general method (2.6.1). Special difficulties arise with radiopharmaceutical preparations because of the short half-life of some radionuclides, the small size of batches and the radiation hazards. In the case that the monograph states that the preparation can be released for use before completion of the test for sterility, the sterility test must be started as soon as practically possible in relation to the radiation. If not started immediately, samples are stored under conditions that are shown to be appropriate in order to prevent false negative results. Parametric release (5.1.1) of the product manufactured by a fully validated process is the method of choice in such cases. When aseptic manufacturing is used, the test for sterility has to be performed as a control of the quality of production.
When the size of a batch of the radiopharmaceutical preparation is limited to 1 or a few samples, sampling the batch for sterility testing according to the recommendations of the general method (2.6.1) may not be applicable.
When the half-life of the radionuclide is less than 5 min, the administration of the radiopharmaceutical preparation to the patient is generally on-line with a validated production system.
For safety reasons (high level of radioactivity) it is not possible to use the quantity of radiopharmaceutical preparations as required in the test for sterility (2.6.1). The method of membrane filtration is preferred to limit irradiation of personnel.
Notwithstanding the requirements concerning the use of antimicrobial preservatives in the monograph Parenteral preparations (0520), their addition to radiopharmaceutical preparations in multidose containers is not obligatory, unless prescribed in the monograph.
Bacterial endotoxins - pyrogens
Radiopharmaceuticals for parenteral administration comply with the test for bacterial endotoxins (2.6.14) or with the test for pyrogens (2.6.8).
Eluates of radionuclide generators, solutions for radiolabelling and kits for radiopharmaceutical preparations also comply with the test for bacterial endotoxins if they are intended for the preparation of radiopharmaceuticals for parenteral administration without further purification.
Radionuclide precursors comply with the test for bacterial endotoxins if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins.
The test for bacterial endotoxins is carried out as described in the general method (2.6.14), taking the necessary precautions to limit irradiation of the personnel carrying out the test. The limit for bacterial endotoxins is indicated in the individual monograph or calculated according to general chapter 5.1.10. Guidelines for using the test for bacterial endotoxins.
When the nature of the radiopharmaceutical preparation results in interference in the test for bacterial endotoxins by inhibition or activation and it is not possible to eliminate the interfering factor(s), the test for pyrogens (2.6.8) may be specifically prescribed.
STORAGE
Store preparations containing radioactive substances in an airtight container that is sufficiently shielded to protect personnel from irradiation by primary or secondary emissions and that complies with national and international regulations concerning the storage of radioactive substances. During storage, containers may darken due to irradiation. Such darkening does not necessarily involve deterioration of the preparations.
LABELLING
The labelling of radiopharmaceutical preparations complies with the relevant national and European legislation.
For preparations prepared at the site of use, the labelling can be modified.
The radioactivity of a preparation is stated at a given date. If the half-life is less than 70 days the time is also indicated, with reference to a time zone. The radioactivity at other times may be calculated from the decay equation or from tables.
In addition to the above, the label on the container, the package, a leaflet accompanying the package or a certificate of analysis accompanying the radiopharmaceutical preparation states:
DETECTION AND MEASUREMENT OF RADIOACTIVITY
Detection and measurement of radioactivity are carried out according to general chapter 2.2.66. Detection and measurement of radioactivity.
Ph Eur